Implant Card Requirement – A New Requirement of EU 2017/745
This article breaks down and reviews the new implant card requirement as well as Article 18 of EU 2017/745.
We also have available for sale, SYS-037 Implant Card Procedure written to be Article 18 compliant of Regulation (EU) 2017/745, and includes;
- SYS-037 A, Implant Card Procedure
- FRM-044 Checklist for Information to be supplied to the patient with an implant
- FRM-045 Implant Card Checklist for Article 18 Reg 2017-745
- Native Slide Deck for Implant Card Webinar
- Recording of the Implant Card Webinar

Implant Card Requirement, a new requirement from Regulation (EU) 2017/745.
One of the new changes to the regulation is an introduction of a new requirement for implantable devices. These devices must now come with an “implant card” that contains information about the implanted medical device for the patient. The responsibility of the implementation of the new implant card rules lies with the manufacturer of the implantable device and the health institution as required by the EU member states.
What is an implantable device?
Before discussing the specifics of the implant card, we must first define what an implantable device is to determine if the implant card requirements apply to your device or devices. Article 2 Definitions, number 5 of Regulation (EU) 2017/745 defines and outlines what is considered an implantable device.
“(5) ‘implantable device’ means any device, including those that are partially or wholly absorbed, which is intended:
– to be introduced in the human body, or
– to replace an epithelial surface or the surface of the eye,
By clinical intervention and which is intended to remain in place after the procedure.
Any device intended to be partially introduced into the human body by clinical intervention and intended to remain in place after the procedure for at least 30 days shall also be deemed to be an implantable device; “
(Taken from http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0745 English version)
Is my device considered implantable?
Working with the above definition of an implantable device, you can now compare those requirements against your own devices to determine if they are considered to be an implantable device or not. This can be done by performing a gap analysis of the definition against your device.
Consider what your device is and ask yourself the following questions:
Is my device intended to be partially or wholly absorbed?
If the answer is no, then your device may not be an implantable one. If it is, then you must keep asking yourself questions until you can sufficiently determine your device’s status as implantable or not.
Is my device intended to be introduced in the human body?
No. Ok, that is fine, but is it intended to replace an epithelial surface or the surface of the eye?
To make an awful analogy of the process, it is almost like playing a game of Guess Who with your device. Instead of asking your device if they have red hair or a mustache, you have to ask your device questions like, “Are you intended to remain in place after the procedure?”.
The gap analysis is fine, but you also have to consider some other factors within the wording of the definition. Be careful navigating the specifics because the devil is in the details. In the definition, which is only eighty-nine words long, by the way, uses the word “intended” three different times.
That is important because the definition applies not only to some of the characteristics and uses of the device but also to the intent behind the device. Just because the device can be wholly introduced into the body does not mean that the device is ‘intended’ to be. A better example would be, by clinical intervention, can your device remain in place after the procedure? Could it, perhaps, but is it intended to be? Also, is it the intent of the device to be done so by clinical intervention?
Where to find the implant card requirement?
Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices is where the introduction of implant cards can be found. The definition of an implantable device is found in Article 2 Definitions, definition number 5.
Article 18- ‘Implant card and information to be supplied to the patient with an implanted device’ is where the implant card requirements can be found. This article contains three sections and four subsections pertaining to implant cards.
Article 18 Implant card requirement and information to be supplied to the patient with an implanted device
Below is article 18 in its entirety so that we can discuss it further in detail.
“1. The manufacturer of an implantable device shall provide together with the device the following:
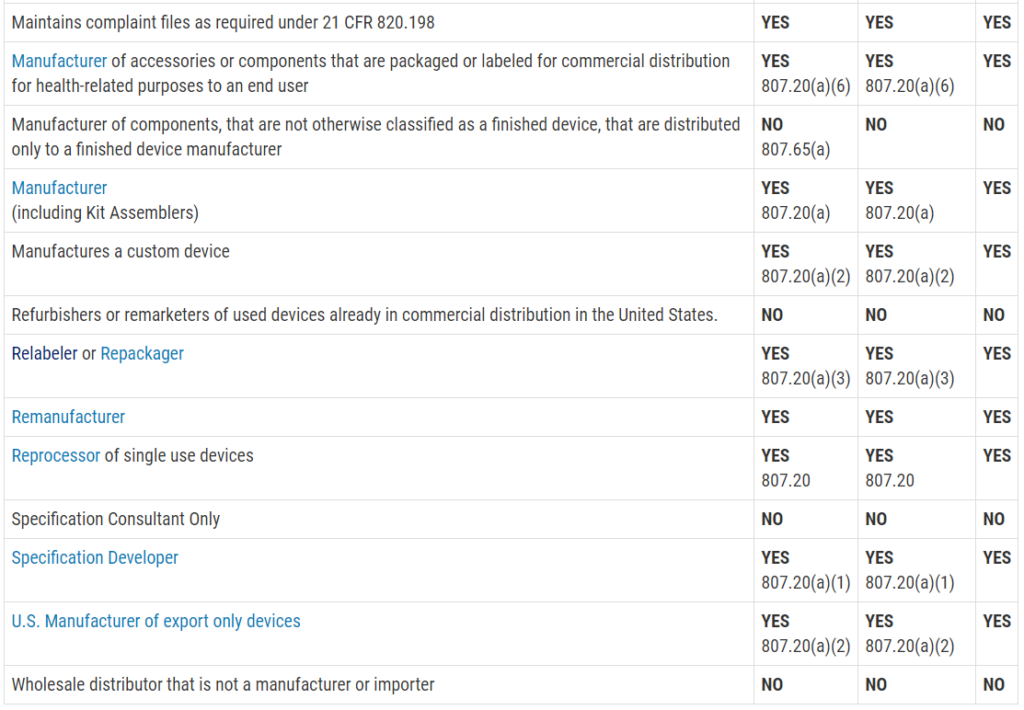
| (a) | information allowing the identification of the device, including the device name, serial number, lot number, the UDI, the device model, as well as the name, address and the website of the manufacturer; |
| (b) | any warnings, precautions or measures to be taken by the patient or a healthcare professional with regard to reciprocal interference with reasonably foreseeable external influences, medical examinations or environmental conditions; |
| (c) | any information about the expected lifetime of the device and any necessary follow-up; |
| (d) | any other information to ensure the safe use of the device by the patient, including the information in point (u) of Section 23.4 of Annex I. |
The information referred to in the first subparagraph shall be provided, to make it available to the particular patient who has been implanted with the device, by any means that allow rapid access to that information and shall be stated in the language(s) determined by the concerned Member State. The information shall be written in a way that is readily understood by a layperson and shall be updated where appropriate. Updates of the information shall be made available to the patient via the website mentioned in point (a) of the first subparagraph.
Also, the manufacturer shall provide the information referred to in point (a) of the first subparagraph on an implant card delivered with the device.
- The Member States shall require health institutions to make the information referred to in paragraph 1 available, by any means that allow rapid access to that information, to any patients who have been implanted with the device, together with the implant card, which shall bear their identity.
- The following implants shall be exempted from the obligations laid down in this Article: sutures, staples, dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips, and connectors. The Commission is empowered to adopt delegated acts in accordance with Article 115 to amend this list by adding other types of implants to it or by removing implants therefrom.”
(taken from http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0745)
Who does the implant card requirement apply to?
Section 1. of Article 18 states explicitly that it is the manufacturer who shall supply the information. Fortunately, it is also outlined what information needs to be included and some guidance on how to provide the information.
Take note, though, that the article states it “shall” be provided, “together with the device.” This means that merely having the information available or accessible such as a downloaded PDF on your website, is not sufficient to comply with section 1. Because that is not being supplied together with the device as outlined.
Section 2. of Article 18 applies to member states’ requirements of health care institutions.
Section 1 of Article 18
Section 1 is by far the most extended section of the article and outlines precisely what information must be provided with the implantable device. Not only is this information that must be provided, it specifically must be provided by the manufacturer. The subsections are broken down by topic and can be summarized as the information, warning, maintenance, and misc. Sections.
Section 1. Sub-Section A
This sub-section outlines the specific identifying information that must be provided. It is even specifically “information allowing the identification of the device.” For devices that are produced and manufactured compliant with other standards such as ISO 13485 or the QSR portion of the United States Code of Federal Regulations, a lot of this information is the same information that is required for traceability.
Besides the generic “information allowing the identification of the device,” the other specific information that ‘shall’ be provided is:
- The name of the device,
- The device serial number,
- The lot number of the device,
- The UDI,
- The model of the device,
- The name of the manufacturer,
- The manufacturers address,
- The manufacturers’ website.
They don’t just want your device’s driver’s license; they want the driver’s license, library card, passport, blood type, and favorite color. This is done for a purpose but also carries some implications on the maintenance actions of the manufacturer.
First such strict ID requirements mean that the device is traceable and identifiable. There should be absolutely no doubt about who made the device. In the event of an incident, that device should be traceable back to when and where the individual components were created and assembled into the final device. For traceability of an incident, tracking for corrective or preventive action, or just general inventory tracking this is the type of strict diligence that is expected when the end-user or patient is receiving medical care with an implantable device. There is no demonizing of this requirement. Yes, it is strict, but it is also just part of good housekeeping for a manufacturer in general. Only now it must be provided to the patient receiving care with the device as well.
What is implied is that the information provided along with the device is somewhat of a living document, and the information could vary a bit from patient to patient. Because things like lot numbers or any number of trackable metrics used with the UDI are included, the implant card information cannot be generically the same for each device but that it will have sections that are specific to individual devices. Sure this may initially create some logistical headaches for keeping track that the implant cards don’t get mixed up in situations where the devices are being manufactured, but this creates a level of accountability that is designed for the ultimate safety of the end patient.
Section 1. Sub-section B
Sub-section B contains the warning information of the device. The first part is pretty self-explanatory as meaning literally what is stated “any warnings” and “precautions”. It is the next part that I do not interpret literally. Where it says “measures to be taken by the patient or a healthcare professional with regard to reciprocal interference with reasonably foreseeable external influences, medical examinations or environmental conditions”.
If I were the manufacture of an implantable medical device, I would most definitely include measures to be taken by the patient as well as measures to be taken by a healthcare professional. There are a couple of spots that use the word ‘or’, and if it were me, I would read it ‘as well as’.
I say that for a few reasons. One is that without explicit clarification of a governing body as exactly what a silly little word like that is intended to me, this creates an area that is open for debate. Does that ‘or’ mean that at least one of those needs to be included and the rest can be excluded?
As one who likes to err on the side of caution, if you have the information available, why would you not provide it? By going above and beyond not only demonstrates your goodwill but also avoids hang-ups where an auditor might not agree with how you viewed the requirement, and you end up with a nonconformity, or in the same situation with an incident investigator. Ink is cheap; liabilities are expensive.
Section 1. Sub-section C, and Sub-section D.
These two subsections are relatively short and straight forward.
“(c) any information about the expected lifetime of the device and any necessary follow-up;
How long can the user expect your device to last once it has been implanted? I there any maintenance they should be performed? Perhaps once a year, a physician needs to double-check the device placement?
(d) any other information to ensure the safe use of the device by the patient, including the information in point (u) of Section 23.4 of Annex I.”
The rest of Section 1. Of Article 18.
“The information referred to in the first subparagraph shall be provided, to make it available to the particular patient who has been implanted with the device, by any means that allow rapid access to that information and shall be stated in the language(s) determined by the concerned Member State. The information shall be written in a way that is readily understood by a layperson and shall be updated where appropriate. Updates of the information shall be made available to the patient via the website mentioned in point (a) of the first subparagraph.
Also, the manufacturer shall provide the information referred to in point (a) of the first subparagraph on an implant card delivered with the device.”
(Taken from http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0745 English version)
At the end of this section, it provides a little bit more information about the purpose of the article but also lays out some guidelines for how to make the required information available.
I specifically mentioned earlier that having the information slapped on a website is not enough by itself. The text states, “any means that allow rapid access to that information”. Certainly, available on the internet is a means that allows rapid access, and it is if you have internet. Using a web-based approach like that is assuming that all the possible patients all have the technology and budget to reach the information. This means that every single possible patient needs a means to access the internet, and the money to pay for internet access. Also, being able to simply access the information rapidly isn’t necessarily providing the information “together with the device” as required.
You also need to have a conversation with your notified body and determine what languages are required by the member state in which your device is sold. It does not do the patient much good if they do not understand the language in which the information is being presented. It also needs to be presented in easy to understand terms, not in technical jargon.
Updates, unlike the initial presentation of information, needs to be included on your website. Specifically, the website that was included in the implant card given to the patient.
Section 2. of Article 18
Unlike what we saw in Section 1. Section 2. Outlines requirements for the health institutions and not the manufacturer. More specifically, Section 2. Requires member states to require health institutions to perform actions.
This section makes health institutions provide the same information that manufacturers had to provide to patients who have been implanted with a device, with the same stipulations as to how the information is provided. However, it also includes the health institution to include their identity on the implant card as well.
- Member States shall require health institutions to make the information referred to in paragraph 1 available, by any means that allow rapid access to that information, to any patients who have been implanted with the device, together with the implant card, which shall bear their identity.
(Taken from http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0745 English version)
Exemptions allowed in Article 18.
Section 3 of Article 18 is the list of exempted implants, exempted devices are:
- Sutures
- Staples
- Dental Fillings
- Dental Braces
- Tooth Crowns
- Screws
- Wedges
- Plates
- Wires
- Pins
- Clips
This is not an exhaustive list and can change with time at the discretion of the Commission. What it has done is taken implanted devices and exempted some of the most common and widely used ones. Thankfully so too, imagine if every staple needed an implant card to be presented to the receiving patient with individual batch and identifying numbers. Then coordinate the effort with a health institution so that the card also bears their identification as well. This would quickly become exhaustive.
- The following implants shall be exempted from the obligations laid down in this Article: sutures, staples, dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips, and connectors. The Commission is empowered to adopt delegated acts in accordance with Article 115 to amend this list by adding other types of implants to it or by removing implants therefrom.”
(Taken from http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0745 English version)
Implant Card Requirement – A New Requirement of EU 2017/745 Read More »