Medical Device Academy is rebooting its medical device and SaMD lead auditor training course, scheduled for release on December 19, 2025.
What is a medical device lead auditor training course?
Auditor training teaches people how to evaluate the effectiveness of a quality system. Most training and quality system standards are based on the original quality system standard, ISO 9001, that was released in 1994. The lead auditor has additional responsibilities, including managing an audit program, planning the audit schedule, assigning auditors, and overseeing the audit team. A medical device lead auditor receives specialized training based on the ISO 13485 quality system standard and requires additional training on processes specific to the design, manufacture, and distribution of medical devices. Medical device lead auditor training for quality systems has been around since 1996, when the ISO 13485 standard was introduced. Unfortunately, some training companies are still using content Rob Packard helped create in 2010 (or even older content). Lead auditor courses include basic concepts:
- Audit Preparation & Planning (1st, 2nd, 3rd party audits)
- How to Conduct Opening & Closing Meetings
- Interviewing Techniques
- Note Taking
- Audit Team Management
- Audit Program Planning
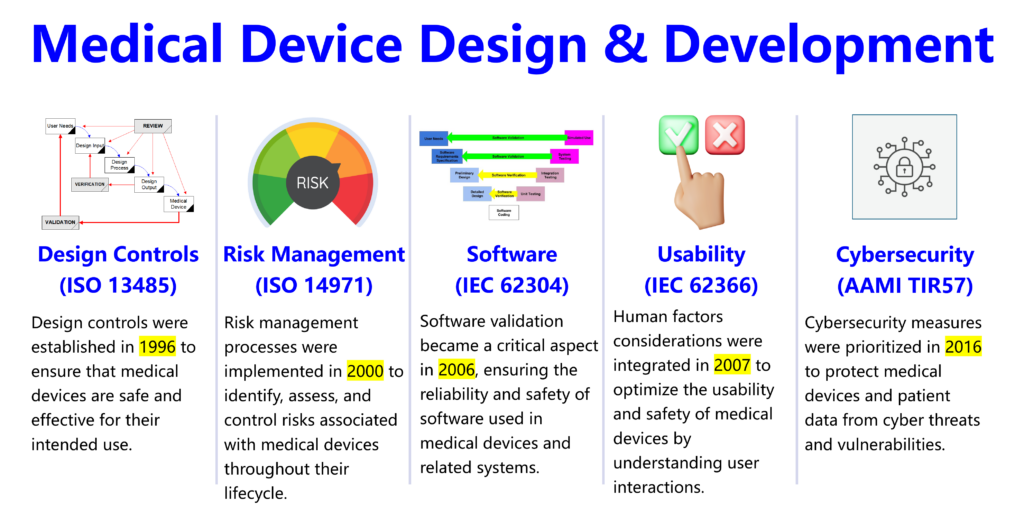
Medical Device Academy’s lead auditor course also covers these basic concepts, but we also introduce modern medical device topics (e.g., risk management, software, usability, and cybersecurity) that were non-existent prior to 2000.
Keep learning fun and engaging
The best courses include role-playing to keep it fun and entertaining while helping students visualize a real audit. Unfortunately, role-playing is not feasible in a 100% online and on-demand environment. To help students visualize that role-playing in an online and on-demand environment, our rebooted course utilizes videos where our scrum team demonstrates auditor best practices and mistakes. Our videos also show you what kind of challenges to expect from an auditee. We also provide examples of procedures and records. We teach multiple methods of auditing–including best practices for the use of checklists and how to eliminate the need for checklists using the process approach to auditing. We even provide tutorials on how to write nonconformities, report writing, and audit follow-up.

We promise to keep learning fun and entertaining
The rebooted lead auditor training course comprises 32 lessons, each of which can be completed in 30 minutes or less. For maximum retention and learning effectiveness, we recommend completing one lesson each business day and taking the lessons in their numbered order (i.e., 1 through 32). If you have prior auditor training, you can take these lessons out of order or even skip content. You can also repeat any lesson at any time as a refresher. You will also have access to future updates at no additional cost. We have a short “pop quiz” at the end of each lesson and a final exam. If you need an updated PDF training certificate (e.g., for refresher training), you only need to repeat the current version of our lead auditor training final exam. The final exam is automatically graded, and training certificates are issued automatically upon completion of the final exam.
What will be included in the course content?
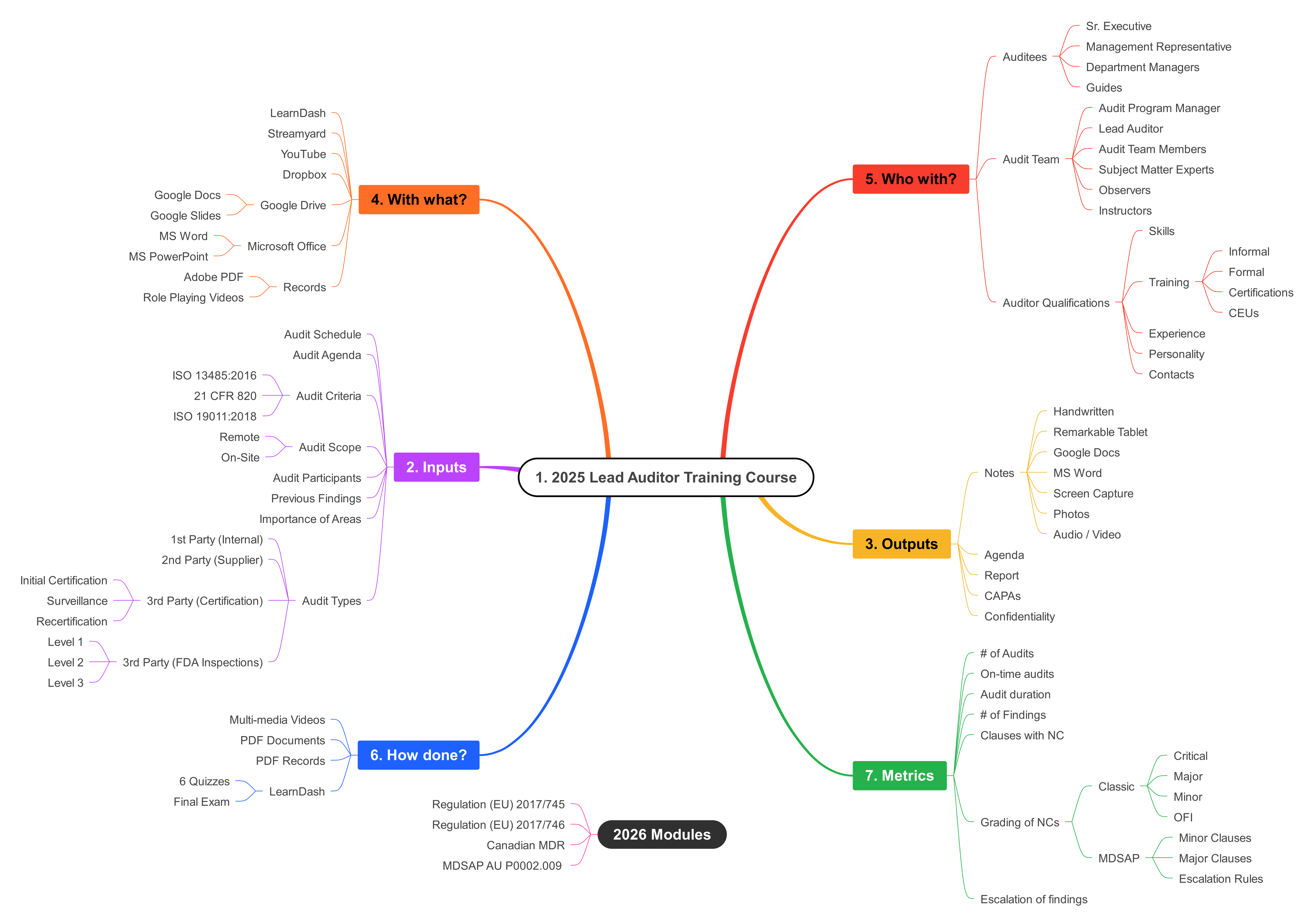
Below we have a mind map that we created to help us brainstorm ideas for the reboot of our lead auditor training course. Not everything in the mind map will be included in the course, but we will cover the following elements to ensure that you are adequately trained as a lead auditor and you understand the basics of software as a medical device (SaMD):
- EN ISO 19011:2018, Guidelines for Auditing Management Systems
- EN ISO 13485:2016+A11:2021, Medical devices – Quality management systems
- 21 CFR 820 – FDA Quality Management System Regulations (updated for February 2, 2026)
- Overview of EN ISO 14971:2019+A11:2021 – Medical devices – Application of risk management to medical devices
- Overview of EN EN 62304:2006+A1:2015 – Medical device software – Software life cycle processes
- Overview of EN 62366-1:2015+A1:2020 – Medical devices – Part 1: Application of usability engineering to medical devices
- Overview of AAMI TIR57:2016 – Principles for Medical Device Security – Risk Management
Why is Lead Auditor Training Online?
Rob Packard previously (i.e., 2009-2019) taught lead auditor training courses as an on-site, live training course for private companies and publicly for small groups up to ten people. Post-pandemic, everyone wants to learn remotely to avoid the hassles of traveling. We also see many more companies choosing to conduct quality system audits remotely. Very few courses include methods for remote auditing, except for one created by Medical Device Academy for a non-profit several years ago. Since the entire team at Medical Device Academy already works 100% remotely and conducts audits remotely, there is no other team more experienced in conducting remote audits and remote training. Our consulting firm has clients worldwide, and we know firsthand how exhausting it is to conduct virtual meetings every day with people working in Korea, Europe, and the United States. You want a high-quality, engaging training course that is fun and effective. It’s the responsibility of our instructors to ensure that learning is both fun and effective, despite being delivered online and on demand.
When will the online lead auditor training launch?
The medical device and SaMD lead auditor training course is scheduled for release on Friday, December 19, 2025, at 5:00 p.m. ET. The course is 100% on-demand. Therefore, you can start and finish the course at 5:01 p.m. ET, or anytime after that. The lead auditor training is also self-paced. If you want to finish the course as quickly as possible, you could complete the lead auditor training before Monday, December 22, 2025. For those with less urgent training needs, if you follow our recommended training cadence of one lesson per day (5 days per week), you can finish the course as early as February 3, 2026 (i.e., 32 lessons, Monday through Friday). We expect most people to skip a few days, but to maintain this new training habit, we recommend never skipping more than one business day in a row.
How much will it cost?
The lead auditor training course will be offered as a pre-order on December 1, 2025, for $1,900 per person. After the scheduled launch on December 19, the price will increase to $2,600. All updates to the course are free to the original subscriber. If you would like to subscribe to our preorder list to receive additional notifications about this lead auditor course, please fill in the form below. There is a dropdown selection for anyone who would like to volunteer to participate as a Key Stakeholder in one of our Sprint Reviews. We will select some a few volunteers to meet with our Scrum team virtually, receive a “sneak peek” of two new lessons we create, and provide feedback on proposed lessons in our Backlog. The time commitment will be approximately one hour.
Reference Materials Needed:
Students will also need access to a copy of the following documents:
- EN ISO 19011:2018 (you can purchase at https://evs.ee/)
- EN ISO 13485:2016+A11:2021 (you can purchase at https://evs.ee/)
- EN 62304:2006+A1:2015 (you can purchase at https://evs.ee/)
- EN ISO 14971:2019+A11:2021 (you can purchase at https://evs.ee/)
- AAMI TIR57:2016 (you can purchase at https://webstore.ansi.org/)
- EN 62366-1:2015+A1:2020 (your can purchase at https://evs.ee/)
The online lead auditor training course is self-paced, 100% virtual, and available on demand. The course requires prior knowledge of ISO 13485:2016, but Medical Device Academy has ISO 13485:2016 training webinar available on demand as well.
Does Medical Device Academy also offer on-site training or corporate rates?
We also offer live, on-site training courses for lead auditors. The on-site training is four days in length, consisting of eight lessons per day. Every student in a live course can answer the “pop quiz” at the end of each lesson–even if they are participating remotely. They can also make up for missed lessons by watching the on-demand lessons. At the end of the course, all participants are provided with a link to an online final exam, which is automatically graded. Training certificates are automatically issued as a PDF. For a quote on the on-site auditing course or corporate rates, please contact Lindsey Walker. She can also provide a quote for remote or on-site auditing.
Lindsey Walker, Director of Sales
 Lindsey Walker studied at Castleton University, way back when it was just a little old Castleton State College in Castleton, Vermont, where she received her BS in Business Marketing. She also studied at North Country Community College, where she received her Certificate in Practical Nursing. Besides preparing proposals and sending out invoices, Lindsey was recently promoted to Director of Sales. In this new position, she is responsible for managing the sales team, coordinating introductory calls with our clients, creating proposals, and managing our new billing clerk. Lindsey loves cars, but when she is not behind the wheel of one, you can find her on a pottery wheel.
Lindsey Walker studied at Castleton University, way back when it was just a little old Castleton State College in Castleton, Vermont, where she received her BS in Business Marketing. She also studied at North Country Community College, where she received her Certificate in Practical Nursing. Besides preparing proposals and sending out invoices, Lindsey was recently promoted to Director of Sales. In this new position, she is responsible for managing the sales team, coordinating introductory calls with our clients, creating proposals, and managing our new billing clerk. Lindsey loves cars, but when she is not behind the wheel of one, you can find her on a pottery wheel.
Email | lindsey@medicaldeviceacademy.com Tel | (802) 989-3939
Your Lead Auditor Training Course Instructors:
Two of our past instructors are listed below, but this reboot of the lead auditor training course will be revised and updated by our Scrum team.
Rob Packard
Rob Packard is a regulatory consultant with ~25 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Rob was a senior manager at several medical device companies, including the President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009 to 2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Rob’s specialty is regulatory submissions for high-risk medical devices, including implants and drug/device combination products for CE marking applications, Canadian medical device license applications, and 510(k) submissions. The favorite part of his job is training others. He can be reached via phone at +1.802.258.1881 or by email. You can also follow him on YouTube, LinkedIn, or Twitter.
Matthew Walker
 Matthew came to us with a regulatory background that focused on OSHA and NFPA regulations when he was a Firefighter/EMT. Since we kidnapped him from his other career, he now works in Medical Device Quality Management Systems, Technical/Medical Writing, and is a Lead Auditor. Matthew has updated all of our procedures for He is currently a student in Champlain College’s Cyber Forensics and Digital Investigations program, and we are proud to say that he is also a member of both the Golden Keys and Phi Theta Kappa Honor Societies! Matthew participates as a member of our audit team and has a passion for risk management and human factors engineering. Always the mad scientist, Matthew pairs his professional life in regulatory affairs with hobbies in the culinary arts as he also holds a Butchers/Meat Cutters certificate from Vermont Technical College. He can be reached by email. You can also follow him on LinkedIn or YouTube.
Matthew came to us with a regulatory background that focused on OSHA and NFPA regulations when he was a Firefighter/EMT. Since we kidnapped him from his other career, he now works in Medical Device Quality Management Systems, Technical/Medical Writing, and is a Lead Auditor. Matthew has updated all of our procedures for He is currently a student in Champlain College’s Cyber Forensics and Digital Investigations program, and we are proud to say that he is also a member of both the Golden Keys and Phi Theta Kappa Honor Societies! Matthew participates as a member of our audit team and has a passion for risk management and human factors engineering. Always the mad scientist, Matthew pairs his professional life in regulatory affairs with hobbies in the culinary arts as he also holds a Butchers/Meat Cutters certificate from Vermont Technical College. He can be reached by email. You can also follow him on LinkedIn or YouTube.


