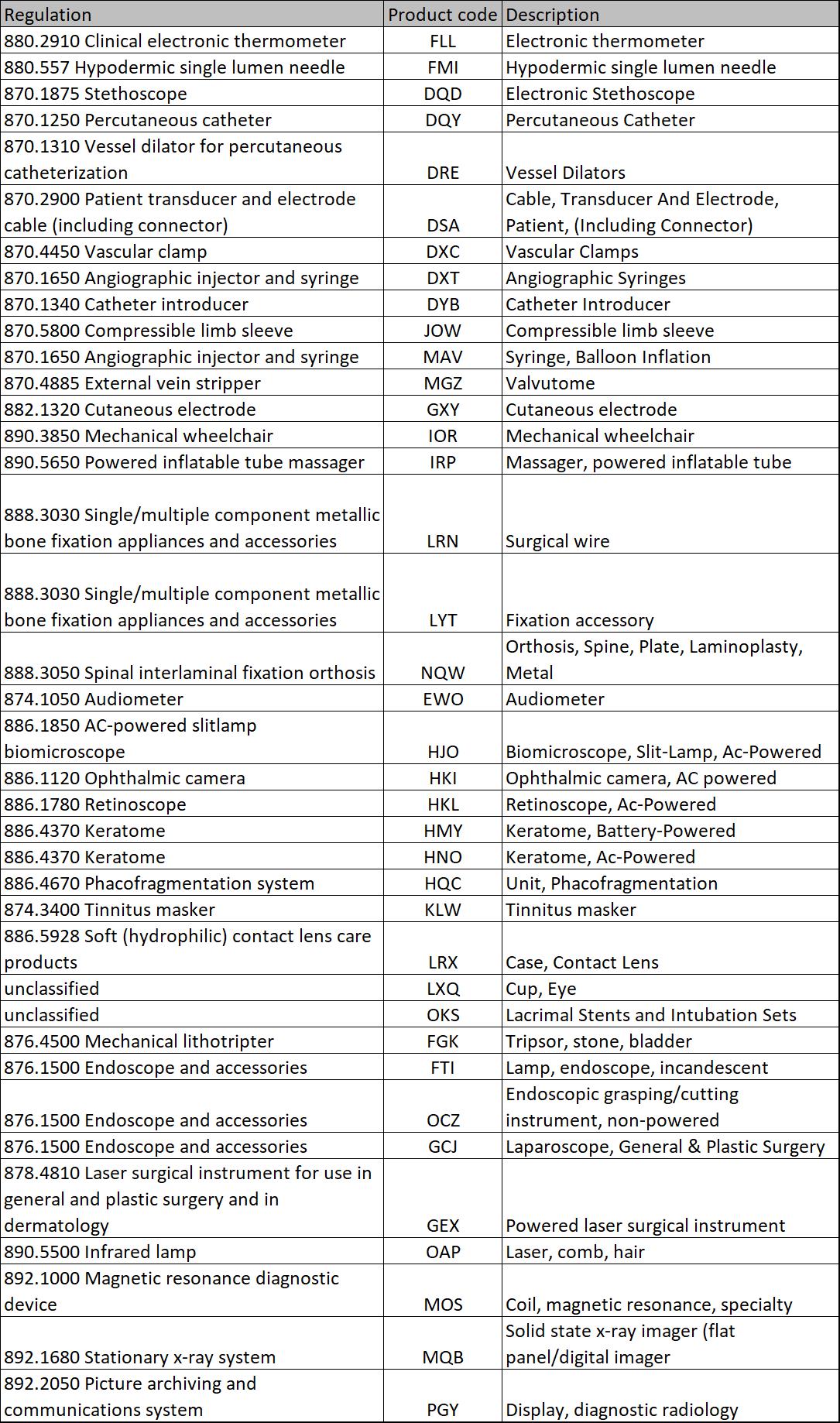
There are 38 product classification codes that the FDA selected for the Quik 510k Pilot program to evaluate version 3 of the eSubmitter software.
What are the three (3) advantages of the new Quik 510k pilot program?
There are three (3) advantages of using the eSubmitter software as part of the Quik 510k pilot. The first advantage of using the eSubmitter software is that the refusal to accept (RTA) process will be eliminated. This change is enormous because nearly 50% of submissions are rejected during the RTA screening process. The hope is that the eSubmitter software will prevent companies from submitting submissions that are missing required content, and therefore the RTA process will not be needed. However, we have seen many submissions placed on hold for technicalities rather than sub-standard submissions. Consequently, it will be fascinating to see the FDA reported outcomes from the Quik 510k pilot.
The second advantage of using the eSubmitter software is that the reviews will be interactive. This means that reviewers are not expected to have any additional information (AI) requests. This also means that submitters will need to respond to questions from reviewers quickly. For example, I have received a call on Friday afternoon after 5:00 pm EDT asking if I could revise to document and email that document to the reviewer by Monday morning. This is an extreme example, but 48-72 hours is typical for a required turn-around during interactive reviews.
The third advantage of using the eSubmitter software is that the FDA is targeting completion of their 510k review within 60 days. This 30-day reduction may seem huge, but the FDA already cut 15 days off its review timeline by eliminating the RTA screening. Second, the FDA picked 38 product classification codes that should not have difficulty reviewing in 60 days. Not all product classifications have the same amount of testing data required, and I do not expect the FDA to be able to review all product classification codes in 60 days–even with eSubmitter.
Although the Quik 510k pilot mentioned that submissions would be zipped, eSubmitter is also designed for electronic submissions through an electronic submissions gateway (ESG). An ESG has the added advantage that you will not need to ship your submission via FedEx. This advantage will gain you only a maximum of 24 hours, but I wish I had those 24 hours last week. Every year, in the last week of September, all the small businesses with small business qualifications try to submit their 510k before the end of the fiscal year (i.e., September 30). This year I had four clients that were in this position. One was unable to get the data they needed to complete their submission before September 30. The other three were making last-minute changes up until the afternoon of Thursday, September 27. One of those submissions was extremely challenging because the submission included video files that exceeded 1GB in total. Therefore, I called CDRH’s eCopy Program Coordinators at 240-402-3717. They were accommodating. They said that it would be best to provide two identical eCopies or to save the MISC FILES and STATISTICAL DATA folders on a separate flash drive. The reason for this is that very large submissions can take days to upload into the CDRH database. Therefore, the picture below shows you what my final solution was for the three submissions this week. The De Novo submission had to be split.

What our firm has done to take advantage of the Quik 510k pilot
If you have a product with any of the 38 product classification codes listed above, and you need to submit a 510k in the next six months, you are very fortunate. The FDA will prioritize your submission, and you are likely to be able to get your device cleared in 60 days or less. Our firm is very anxious to take part in this pilot because the FDA intends to require the eSubmitter software for all submissions in the future, and we expect other product classification codes to be added to the pilot over time. We process dozens of 510k submissions each year, and mastering the nuances of the software is critical to our continued success. I already downloaded the software and installed it onto my computer. I also created a complete submission as a test. eSubmitter saved several hours in the preparation of a 510(k) from the typical 40 hours the process takes. Therefore, I expect the implementation of new eSubmitter software to a triple win for the FDA, clients, and our firm. I plan to request that the FDA add De Novo submissions next to this pilot. The reason is that De Novo submissions typically have more content, and the content is more variable. I think this would be an extremely challenging test for eSubmitter, and the relatively small volume of De Novo submissions would limit the impact upon FDA resources.
Changes to eCopy Requirements in 2018
In 2017, the FDA indicated that eSubmitter software was going to be revised, and it would be approximately two years before companies would be able to submit a 510k electronically to the FDA. Until then, companies must ship an electronic eCopy and a paper copy to the FDA Document Control Center (DCC). The eCopy guidance states, “An eCopy is accompanied by a paper copy of the signed cover letter and the complete paper submission.” However, the FDA’s eCopy guidance has not been updated since December 3, 2015. There are some unofficial changes to the policy, and the FDA no longer requires the complete paper submission. Instead, you can submit an eCopy accompanied by a paper copy of the signed cover letter.
Before February 2018, we would print 1,000+ pages for each 510k submission, pack two 3” three-ring binders in 12”x12”x6” ULine boxes and ship the box to the FDA overnight via FedEx. We typically would charge $400 for this eCopy service. After the unofficial policy change, all of our 510k submissions consist of a paper copy of the cover letter and an eCopy on a USB flash drive. We only charge $150 for the FDA eCopy service, and 100% of our eCopy submissions have been uploaded without problems this year.
What is the difference between creating an eCopy and submitting it with eSubmitter (cited from FDA website)?
There are four differences between eSubmitter and eCopies:
- An eSubmission package contains PDF attachments and XML file types. The XML files are intended for CDRH IT systems to process the application. Reviewers will not see these XML files.
- The parts of the eCopy guidance that describe the structure of a 510(k) submission will not apply to the Quik Review Program Pilot.
- An eSubmission is organized according to the layout of the template, which places administrative documents (e.g., Form 3674, the 510(k) Summary, the Truthful and Accurate statement) at the end of the submission because their applicability is determined based on the answers to questions in the body of the template (e.g., Form 3674 is only required if the applicant indicates clinical data are included).
- Electronic signatures are used in the submission (e.g., on the Truthful and Accurate statement), rather than physical signatures.
eSubmitter Template Options
For device 510k submissions, the FDA’s eSubmitter gives you three options:
- Template Version 1.3, for In Vitro Diagnostic 510k submissions to CDRH only, allows you to create a 510k submission and the eSubmitter software will package your submission in a specially formatted zip folder that you can save to a compact disc (CD), digital video disc (DVD) or flash drive. Then you must print a paper copy of your signed cover letter and ship the eCopy created by eSubmitter with your paper copy of the cover letter to the FDA DCC.
- Template Version 1.2.1, for Non-In Vitro Diagnostic 510k submissions that are among the 1,000+ other product classifications not included in the Quik 510k pilot (CDRH: Medical Device eCopies), you can create a 510k submission and the eSubmitter software will package your submission in a folder for you. You can then copy the contents of that folder to a compact disc (CD), digital video disc (DVD), or flash drive. Then you must print a paper copy of your signed cover letter and ship the eCopy created by eSubmitter with your paper copy of the cover letter to the FDA DCC.
- Template Version 3.2, for Non-In Vitro Diagnostic 510k submissions that are among the 38 product classification codes that are listed above for the Quik 510k pilot program. This allows you to create a 510k submission, and the eSubmitter software will package your submission in a specially formatted zip folder that you can save to a compact disc (CD), digital video disc (DVD), or flash drive. Then you must print a paper copy of your signed cover letter and ship the eCopy created by eSubmitter with your paper copy of the cover letter to the FDA DCC. This template is unique to the Quik 510k pilot program. There is a red bar that appears at the top of the screen:
“This template should only be used to construct a submission if you are submitting it as part of the Quick Review Pilot. All others may use the content of this template as a reference to aid in constructing an eCopy. If you are not part of the Quick Review Pilot, do not construct a submission with this template, it will be rejected.”
When you create your eCopy, then you will need to create a volume-based or non-volume based submission in accordance with the eCopy guidance. The volume folders and/or files are saved to a compact disc (CD), digital video disc (DVD), or flash drive. Then you must print a paper copy of your signed cover letter and ship the eCopy you created with your paper copy of the cover letter to the FDA DCC.
 Warning: If you are using Windows 10, and you save your eCopy or eSubmitter zip folder on a flash drive, Windows 10 will automatically create a hidden system folder titled “System Information Volume.” This folder is created as a security feature to enable you to recover accidentally deleted content. However, this folder results in an error when the FDA attempts to upload your submission automatically. Therefore, you must remove this hidden system folder. Instructions for this can be found on our website page about eCopy hidden system files.
Warning: If you are using Windows 10, and you save your eCopy or eSubmitter zip folder on a flash drive, Windows 10 will automatically create a hidden system folder titled “System Information Volume.” This folder is created as a security feature to enable you to recover accidentally deleted content. However, this folder results in an error when the FDA attempts to upload your submission automatically. Therefore, you must remove this hidden system folder. Instructions for this can be found on our website page about eCopy hidden system files.

Pingback: Alternate 510k Pathway- Safety and Performance Based Pathway Medical Device Academy