Mary Vater is presenting a free 510k Best Practices Webinar on how to apply Design Controls and Risk Management on Wednesday August 16th, 2017 10am – 11am (EDT).
When is the 510k Best Practices Webinar?
This 510k Best Practices webinar was recorded on Wed Aug 16, 2017 10am – 11am (EDT). It’s free to register as long as you ask a question. As new questions are submitted I will create blogs to answer your questions and add the questions to our 510k FAQs page. In order to register for the 510k Best Practices Webinar please fill in and submit the form below. You will receive an email confirmation including instructions on how to view and participate in the webinar via Zoom.
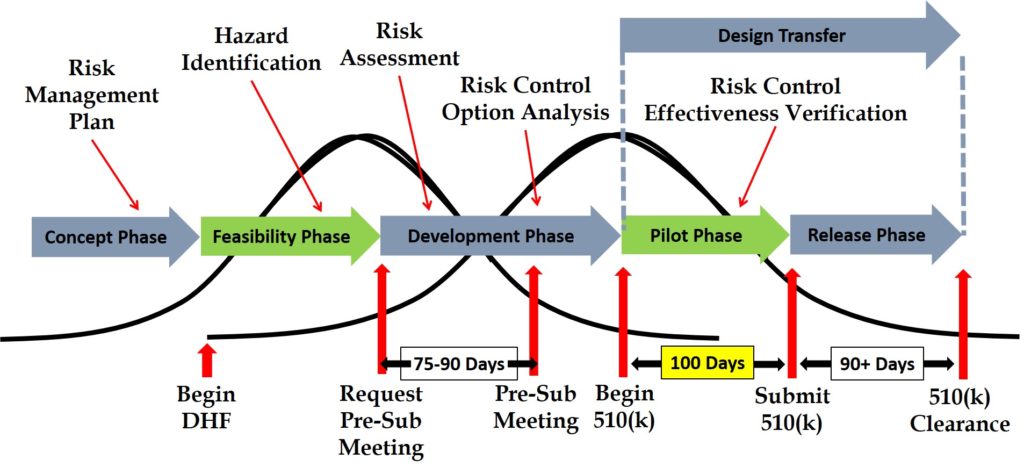
Contents of 510k Best Practices Webinar
Registrants will receive a confirmation email, and then after confirmation, they will receive login information for the live Medical Device Academy webinar. We will also provide a link to download a recording of the webinar and the native slide deck. Additional questions can be asked at any time by sending me an email or scheduling a call on my contact us page.
