This will be the 4th edition of our design controls training webinar with new content to modernize your design and development process.
Design Controls Training
Design controls are the #1 cause of FDA 483s. In this design controls training, you will learn how to avoid an FDA 483 inspection observation. Design controls are 1 of 4 major quality subsystems that the FDA will review during a QSIT level II inspection, and design controls are one of the biggest challenges for companies developing a medical device for the first time. Design controls also apply to any Class 1 device that has software (e.g., MDDS and other software as a medical device or SaMD).
If you sign up now, you can get live access to the design controls training
This design controls training was recorded on Wednesday, August 14, 2024. It was recorded in two parts (~90 minutes each), and the presentation included 44 slides.
Cost is $129 (AND INCLUDES NATIVE SLIDE POWERPOINT PRESENTATION FILES):
Exam and Training Certificate available for $49.00:
What is covered in design controls training?
Our instructor, Rob Packard of Medical Device Academy, reviews basic concepts and requirements of each design phase of the design process from design plan approval through commercial launch approval, including:
- Design planning
- Design inputs
- Design outputs
- Design verification
- Design validation
- Design reviews and more.
Phase/Gate Approach
You will learn how large companies manage product design-related projects (5-6 hard gates) and keep them on schedule. The lifecycle loop (i.e., post-market surveillance, clinical evaluation, and risk analysis) is illustrated below. This post-market surveillance loop illustrates that design and risk management documentation need to be updated continuously and the post-market surveillance is an input to the design control process.
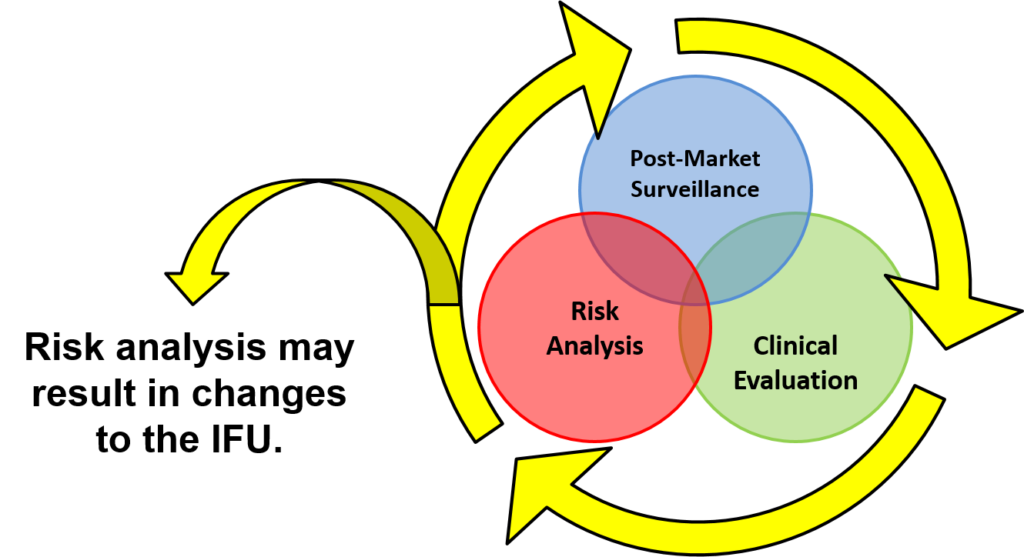
Numerous diagrams are utilized throughout the presentation. Another diagram used in the design controls training shows you how to implement risk management throughout the design process.
The problem with the industry standard approach to the design control process is that it is only meant as a regulatory paperwork exercise. To be an efficient and effective process, design controls need to include documentation that you will actually use. Therefore, in this design controls training you will learn how to add small details that transform ordinary design documents into development tools that you depend upon.
Design Plan
Design plans need to be created earlier and updated often. Project managers don’t like to revise their paperwork, because it takes time. However, the purpose of the design plan is to keep your design team on schedule so that the project is not delayed. Therefore, time spent maintaining your design plan reduces delays and team miscommunications. If you have any team members who are working on their first design project, you should identify that this project will be on-the-job design controls training for them. They will also need risk management training. If you are not sure of a start date or the duration of a task in your plan, then you should have a follow-up action item to clarify the dates and durations. Our webinar will provide several examples.
Design Inputs
Many design teams confuse design inputs with design outputs. The design output is a drawing and/or specification. The design input defines the verification testing that needs to be done and the testing acceptance criteria. Unfortunately, we routinely find that design inputs are documented as a simplified version of the design outputs (e.g., the reusable battery is the input and a specific model of battery is the output). In this design controls training, you will learn a completely different approach using a new development tool. The tool is a form, but this form is designed to help you–not the FDA.
Design Outputs
Engineers love spreadsheets. We even tried to put all of the information you need for a design project in one spreadsheet, but there isn’t enough space in a spreadsheet cell to include all of the information needed. Now we use that spreadsheet as a traceability matrix only. This is an update to your design control procedure (SYS-008) and the design controls training webinar. Design outputs are drawings and specifications. In addition to the design outputs, you will need to generate the bulk of your risk management file, usability engineering file, and your software documentation during the development phase. The development phase ends when you have a “Design Freeze” (i.e., you approve your final design outputs).
Design Verification
Design verification is the testing we do to confirm that the design inputs have been met. Design verification testing is often the most expensive part of the design controls process unless animal studies or human clinical studies are required. The changes we made to design inputs in this design controls training are intended to make design verification progress much faster and without mistakes. Design verification involves creating, reviewing, and approving testing protocols. You have to keep records of the testing results, and then you generate testing reports. Even if you are outsourcing the verification testing, you still need to review and approve the protocols, and you should review the reports to ensure that the necessary information is included. The FDA will require GLP studies for biocompatibility testing because the biocompatibility tests involve small animal testing. During the webinar, we will emphasize the most recent change in required testing from the FDA, and you will learn which tasks are likely to be critical path items that require advanced planning.
Design Validation
Design validation demonstrates that your device can perform the intended use. This typically involves simulated use in a benchtop model, computer analysis (e.g., finite element analysis), cadaver testing, and clinical studies. The human factors process concludes with summative human factors testing. That testing can be considered verification or validation, but the documentation will be attached as non-clinical benchtop performance testing in the FDA eSTAR. The human factors testing can also be done in parallel with human clinical studies using the same subjects. The primary focus of validation testing is demonstrating safety and performance for the intended use, but for FDA 510k submissions you must also demonstrate equivalence to a predicate device. Equivalence may involve side-by-side comparison testing, and you will need to justify your statistical sample size and acceptance criteria for validation testing because there may not be a recognized standard or guidance specifying these requirements.
Software & Cybersecurity Updates for 2023
Software validation requirements were updated by the FDA on June 14, 2023. The applicable changes impact the classification of software and the risk management documentation required for submission. In addition, we expect the FDA to release the final guidance for cybersecurity before the end of September. The new cybersecurity requirements also impact the design plans for any company that is submitting to the FDA.
Updates Regarding Human Factors & Usability Engineering for 2024
We recorded an updated our usability webinar and released a usability procedure (SYS-048). After listening carefully to the webinar and reading through the usability procedure, we updated our combined design/risk management plan to specify formative testing during phase 3 and summative (validation) testing during phase 4 of the design process. You can always modify your own design plans to include this requirement or provide a rationale for why it is not needed for your project. However, my goal was to help explain the value of formative testing and its role relative to summative testing. Minor changes were also made to match the design procedure (SYS-008).
We will also include the impact of the December 2022 draft guidance on Human Factors Engineering. If you are interested in purchasing our usability procedure and template for summative (validation) usability testing, please visit our newest webpage for the usability procedure (SYS-048). Our usability webinar is also available.
Effectiveness of your design controls training and the design control process
At the end of each phase/gate, there is a design review and you should be identifying problems that were encountered and initiate corrective actions when needed. We will conclude our design controls training with recommendations for your review of the design control process. At the end of your design project, during the final design review, you should conduct a more thorough review of the design process to verify that all planned design activities were completed. Any weaknesses in the design process should be evaluated for the need to make changes to your design control procedure and forms, but you may also identify future improvements for design inputs, drawings, specifications, or design review meetings. You should also be scheduling an internal audit of the design control process, and an internal audit of the risk management file after the project is completed to make sure that the design history file (DHF) meets regulatory requirements and the requirements of your own procedure.
VIEW OUR PROCEDURES – CLICK HERE OR IMAGE BELOW:
About your design control instructor
Rob Packard is a regulatory consultant with ~25 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Rob was a senior manager at several medical device companies—including the President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009 to 2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Rob’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510k submissions. The most favorite part of his job is training others. He can be reached via phone at +1.802.258.1881 or by email. You can also follow him on YouTube, LinkedIn, or Twitter.




