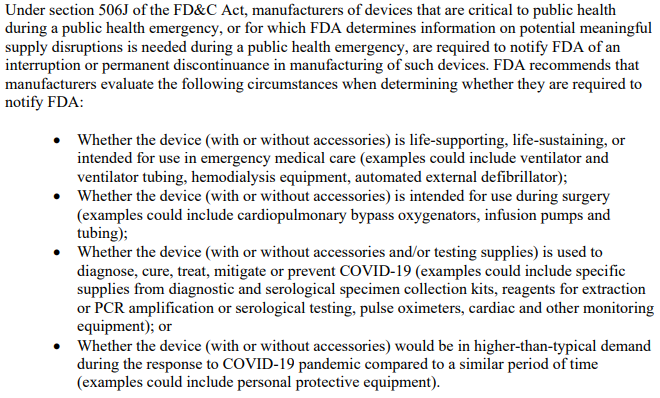
Risk management policy – Do you have one?
ISO 14971:2019 includes a requirement for top management to define and document a risk management policy, but do you have one?

Your risk management procedure is not your risk management policy
ISO 14971:2019 includes a requirement for a risk management policy and a risk management procedure. The word procedure is defined (Clause 3.13), a “specified way to carry out an activity or a process,” but there is no definition for policy. Both of these words begin with the letter “p,” but they are not the same. There is no guidance for a risk management policy in either of the European device regulations for CE Marking and there is no guidance in the US FDA’s regulations. In fact, there is not even a specific cause of the international risk management standard that is specific to the requirement for a risk management policy. The word “policy” only appears in ISO 14971 seven times, but the last occurrence provides the best explanation:
- Appendix A2.4.2 states that “because [ISO 14971] does not define acceptable risk levels, top management is required to establish a policy on how acceptable risks will be determined.”
If someone responsible for risk management activities does not understand this distinction, this shows that risk management training may not be adequate.
Can you have a different policy for each product family?
The purpose of the policy is to establish how the acceptability of risks will be determined. However, not all devices have the same benefit-risk ratio. Therefore, if you have product families with high and low risks, then you should address this in your policy with specific criteria for each device family or create a separate risk management policy for each product family. For example, if your company is focused on designing and developing products for diabetics, you will not have the same benefit-risk profile for a Class 2 glucose reader and lancet for Type 2 diabetics that you have for an automated Class 3 insulin pumps for Type 1 diabetics. In general, separate criteria within one policy are preferred over separate policies to reduce the number of documents that must be managed.
Is there a required format for a risk management policy?
The ISO 14971:2019 standard does not include a specific format or content requirement for your risk management policy. Instead, information about the format and content of a risk management policy is provided in Annex C of ISO/TR 24971:2020. This is a guidance document, and therefore you can choose an alternate approach if you provide a justification for its equivalence. If you choose the approach recommended in Annex C, the following elements should be included:
- purpose;
- scope;
- factors and considerations for determining acceptable risk;
- approaches to risk control;
- requirements for approval and review.
You can download Medical Device Academy’s template for a risk management policy (POL-005) by completing the form below.
What are the factors for determining acceptable risk?
- Applicable regulatory requirements;
- Relevant international standards;
- State-of-the-Art;
- And stakeholder concerns.
An example of regulatory requirements being applied to the determination of acceptable risks is the special controls defined in 21 CFR 880.5730 for insulin pumps. The special controls requirements outlined by the FDA specify design inputs as well as verification and validation requirements. The requirements are also organized into systems that comprise an insulin pump. For the digital interface requirements, the regulation specifies:
- secure pairing to external devices;
- secure data communication between the pump and connected devices;
- sharing of state information between devices;
- ensuring the pump continues to operate safely when receiving data that is outside of the boundary limits that are specified as inputs;
- a detailed process and procedure for sharing pump interface specifications with connected devices.
The hazard implied by the fourth requirement above is that the pump will stop without warning or deliver the incorrect amount of insulin if the data from a continuous glucose sensor is outside of the input specifications. This design input is then addressed by a software design specification established by your company. To verify that your software risk controls are adequate, you will need to execute a verification protocol that automatically inputs a series of values that are outside of the boundary limits specified. Every time a change is made to the software, these boundary limits will need to be re-verified as part of your automated regression analysis to make sure software changes did not have an unintended effect on the device.
For software and use-related hazards, you will not be able to estimate the probability of occurrence of harm. Therefore, you shall assess the acceptability of risks based upon the severity of harm alone. Risk acceptability criteria shall be recorded in your risk management plan and the criteria shall align with your risk management policy. Ideally, these criteria are based upon international standards. For the example of an interoperable insulin pump, the following international standards are applicable:
- ISO 14971, application of risk management to medical devices
- IEC 62366-1, application of usability engineering to medical devices
- IEC 62304, medical device software – software lifecycle processes
For the state-of-the-art, there are three examples provided in the ISO/TR 24971 guidance for how to this relates to your risk management policy:
- “Leakage currents of the medical device are state of the art, demonstrated by compliance to the limits and tests regarding leakage current of IEC 60601-1.
- Dose accuracy of the delivery device are state of the art, as demonstrated by compliance to the limits and tests regarding dose accuracy of ISO 11608-1.
- Protection against mechanical failure caused by impact is on the same level as or better than a similar medical device, as demonstrated by comparative test such as drop test.”
Stake holder concerns is the fourth factor to consider when creating your risk management policy. Stakeholder concerns may be identified in clinical literature. However, the current trend is an emphasis on patient-reported outcome (PRO) data and post-market surveillance. Post-market surveillance is a requirement in ISO 13485, Clause 8.2.1. However, the new European MDR and IVDR have new requirements for post-market surveillance data in the technical documentation. Health Canada updated the medical device regulations to include post-market surveillance summary reports, and even the FDA is trying to develop methods for using real-world data and real-world evidence to make regulatory decisions.
Approaches to risk acceptability
The European device regulations require that a benefit/risk analysis be conducted for all risks and the overall residual risk of your device. The EU regulations also do not permit risk acceptability to consider economic impact. The EU regulations also require that risks are reduced as far as possible. Therefore, if your company is seeking CE Marking, there is only one acceptable approach suggested in ISO/TR 24971, Annex C.2: “reducing risk as far as possible without adversely affecting the benefit-risk ratio.” This is also the approach specified in our risk management procedure (SYS-010).
Requirements for review and approval of the risk policy
Requirements for approval and review of the risk management policy should be specified in the policy itself. This should specify who needs to approve that the policy is acceptable and how often the policy needs to be reviewed. Section 4.2.2 of ISO 14971 also requires that top management review the risk management process for its effectiveness. In general, we recommend that this review of the risk management process be incorporated into the management review process. Therefore, we also believe that this would be the ideal time to review the risk management policy. Generally, this is more frequently than is typically required, but if your risk management process is being reviewed for effectiveness then you have all of the necessary inputs available to review the policy as well.
Risk management policy – Do you have one? Read More »
















 Matthew Walker – QMS, Risk Management, Usability Testing, Cybersecurity
Matthew Walker – QMS, Risk Management, Usability Testing, Cybersecurity