Device Supply Chain Disruptions
What can you do to stay ahead of medical device supply chain disruptions and comply with reporting requirements of possible device shortages?

Supply chain issues can be somewhat cyclical. As we approach the holiday season, we also approach the shipping season. Public shipping services such as FedEx and UPS see an increase in freight as the holiday seasons approach. Manufacturers need raw materials and components to stock the shelves with all of those holiday gifts. Since we are still living under pandemic conditions, I would be willing to bet there will be more care packages and mailed gifts in place of traditional gatherings. On top of the approaching increase in demand, staffing shortages can very quickly exacerbate supply chain bottlenecks. All the while importers are still expected to… well, import! If transportation affects all general industry you can bet it can also cause medical device supply chain disruptions.
So what does an overburdened mail service have to do with medical devices and quality systems?
Consider, how are your customers getting your product in their hands? How are you receiving raw materials and components? How about your contract manufacturer? Do they have supply chain redundancies? Does your supplier quality agreement address notifications for shipping disruptions?
Do you have a regulatory obligation to report a shortage/supply chain disruption or interruption of manufacturing to the FDA, or Health Canada? The FDA monitors for discontinuance and meaningful disruption of manufacturing certain devices and similarly Health Canada monitors their own list of devices for market shortages. Supply chain disruptions either through difficulty sourcing of raw materials and components, or through transportation breakdown of finished devices to market are just one way you could experience a reportable disruption or shortage.
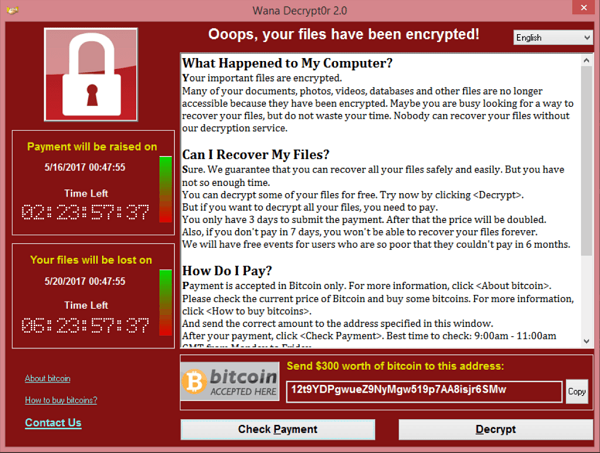
Matthew did not choose the topic of medical device supply chain disruptions randomly. His signature brand of pessimistic cynicism is the reason we have him tasked with keeping his fingers on the pulse of global concerns and potential threats and risks. Potential supply chain disruptions will involve your quality staff in developing preventive actions and contingency plans in case there is an issue. Then, your regulatory team will be in charge of reporting and AHJ notification if you are an affected manufacturer (or importer in Canada!). Understaffed and overloaded shipping and transportation suppliers are about to be bombarded with seasonal freight. This makes them an attractive target for ransomware because, just like healthcare facilities, they will not be in a situation where they can afford any downtime.
Regulatory responsibilities related to device supply chain disruptions – Organized by Jurisdiction

The FDA requires reporting shortages and supply chain disruptions to CDHR of permanent discontinuance or interruption in manufacturing of a medical device in Section 506J of the FD&C Act. Especially so in response to the COVID-19 public health emergency. In part, the general public’s need for healthcare during the pandemic guides what devices the FDA needs notification about.
Currently, the FDA is concerned about specific device types by product code or any devices that are critical to public health during a public health emergency. For the most up to date list, the URL to the FDA website will show the specific product codes of the monitored device types;

As an Authority Having Jurisdiction, Health Canada also has reporting requirements for supply chain disruptions of specific types of medical devices. Health Canada is also an independent authority that uses a different device classification system than the U.S. FDA.
The table below shows the device types by their classification level that HC requires supply chain disruption notifications for. This information is current as of September 5th, 2021, and the following link will take you to the HC webpage for the most up-to-date list.
| Class I Medical Devices |
| Masks (surgical, procedure or medical masks) – Level 1, 2, 3 (ATSM) |
| N95 respirators for medical use |
| KN95 respirators for medical use |
| Face shields |
| Gowns (isolation or surgical gowns) – Level 2, 3 and 4 |
| Gowns (chemotherapy gowns) |
| Class II Medical Devices |
| Ventilators (including bi-level positive airway pressure or BiPAP machines, and continuous positive airway pressure or CPAP machines) |
| Infrared thermometers |
| Digital thermometers |
| Oxygen Concentrators |
| Pulse Oximeters (single measurement) |
| Aspirators/suction pumps (portable and stationary) |
| Laryngoscopes |
| Endotracheal tubes |
| Manual resuscitation bags (individually or part of a kit) |
| Medical Gloves – Examination and Surgical (Nitrile, Vinyl) |
| Oxygen Delivery Devices |
| Class III Medical Devices |
| Ventilators (including bi-level positive airway pressure or BiPAP machines) |
| Pulse Oximeters (continuous monitoring) |
| Vital Signs Monitors |
| Dialyzers |
| Infusion Pumps |
| Anesthesia Delivery Devices |
| Class IV Medical Devices |
| Extracorporeal Membrane Oxygenation (ECMO) Devices |
How to prevent device supply chain disruptions
Harden your supply chain with redundancies. Now is the time to qualify a second supplier as a contingency plan before it is too late…. Maybe even consider opening a Preventive Action? (HINT HINT for those ISO 13485 manufacturers that need to beef up their Clause 8.5.3. operations!)
Supply chains have both up and downstream functions. First, you likely need to source raw materials and components for production. Then you also need to ship those finished devices to distribution centers and your customers. Disrupt either of those and your ability to sell your devices is compromised or even completely halted.
Ask yourself, “Do I have a backup option for shipping?”, and “Do I have a backup option for raw materials and components?”.
Why?
Why go through all of that effort? Well, if you lose UPS and have to use FedEx instead, are their shipping procedures identical? Likely you will need a WI level document for each shipper to explain the process. It is easier to pre-qualify a contingency supplier and establish a WI now rather than in December when holiday shipping is at its peak. Consider if you also need to open accounts, etc. Scheduling pickup online may not be intuitive.
Just identifying a backup is important, but you can take that a step further and pre-qualify them. If they are a shipping and transportation supplier then give them a shipment or two in order to evaluate them. Hold them to the same standards you would for your primary supplier.
Did your shipment arrive on time? Was it damaged during transit? This is provisional, or pre-qualification. Did they perform adequately enough to use as a tentative supplier in the event the primary supplier is unable to perform? This is designed to make a full qualification of this supplier simple and easy… If you need to utilize them that is. Maintaining this pre-qualification should also be simple and easy as well. Once a year or so have them deliver a shipment for you.
That is just for importing or shipping finished devices. Do you have backup raw material or components suppliers identified? If not identifying or even pre-qualifying secondary suppliers might not be a bad idea either. You are probably tied down to a specific geographic area for shipping and transportation. You may not be for raw materials. If you need barrels of silicone consider a backup supplier from a different area than your primary supplier. Natural disasters create havoc for shipping. If your silicone comes from Company A, and they are closed down because of a hurricane then Company B ten miles away is likely affected as well.
For example, if you are in the U.S. and your primary supplier is in the Northeast then a backup supplier in the Southeast may be strategically important. Whereas a backup supplier from the Southwest may be cost-prohibitive.
What about your suppliers? Is your device high-risk enough that if your supply chain is disrupted, you have an obligation to report it to the FDA? In that scenario, if you use a contract manufacturer, it may be worth requiring supply chain contingencies and clearly identifying who owns what reporting responsibilities within your quality agreement with them.
There is an element of proactive responsibility in reporting these shortages, or projected shortages. In order to be able to predict medical device supply chain disruptions, there should be metrics that your quality system is monitoring. What is your monthly production capacity? How much raw material or components does your warehousing have on hand? How many units could you manufacture if the transport industry stopped right this second?
Determine what you need to track in order to identify a disruption before it occurs.
Prepare for notification now. This article looked at the problem from the point of view that transportation issues were the root cause of the supply chain disruption. However, many other things could be disruptive, such as natural disasters and supply availability. Therefore, develop a WI level document for conducting these types of regulatory reporting activities and train personnel before a disruption happens. It is easier to tackle these kinds of problems if you already have process controls in place and trained competent staff than if you wait until the reporting timeline clock is already ticking.
In the near future, we will be posting a new blog about 506J and Shortage Reporting. We will also have a work instruction and training webinar available soon.
Future blogs about device supply chain disruptions…Shortage Reporting
About the Author
 Matthew came to us with a regulatory background that focused on OSHA and NFPA regulations when he was a Firefighter/EMT. Since we kidnapped him from his other career, he now works in Medical Device Quality Management Systems, Technical/Medical Writing, and is a Lead Auditor. Matthew has updated all of our procedures for He is currently a student in Champlain College’s Cybersecurity and Digital Forensics program, and we are proud to say that he is also a member of both the Golden Keys and Phi Theta Kappa Honor Societies! Matthew participates as a member of our audit team and has a passion for risk management and human factors engineering. Always the mad scientist, Matthew pairs his professional life in regulatory affairs with hobbies in the culinary arts as he also holds a Butchers/Meat Cutters certificate from Vermont Technical College.
Matthew came to us with a regulatory background that focused on OSHA and NFPA regulations when he was a Firefighter/EMT. Since we kidnapped him from his other career, he now works in Medical Device Quality Management Systems, Technical/Medical Writing, and is a Lead Auditor. Matthew has updated all of our procedures for He is currently a student in Champlain College’s Cybersecurity and Digital Forensics program, and we are proud to say that he is also a member of both the Golden Keys and Phi Theta Kappa Honor Societies! Matthew participates as a member of our audit team and has a passion for risk management and human factors engineering. Always the mad scientist, Matthew pairs his professional life in regulatory affairs with hobbies in the culinary arts as he also holds a Butchers/Meat Cutters certificate from Vermont Technical College.
Email: Matthew@FDAeCopy.com
Connect on Linkedin: http://www.linkedin.com/in/matthew-walker-214718101/
Device Supply Chain Disruptions Read More »



 In this text box, we need you to identify the process areas you want us to audit. You can ask us to audit just one process or multiple processes. For example, if you are the Quality Manager and the only qualified lead auditor in your company, you might want us to audit your internal auditing, CAPA, management review, control of documents, and control of records. For a single process audit, we generally recommend remote audits via Zoom in order to eliminate the cost of travel. This is also a great way to test us before you engage our firm for a full-quality system internal audit. This is also known as the “audit scope,” and should not be confused with “audit criteria” discussed below. The scope can also include the location of the audit.
In this text box, we need you to identify the process areas you want us to audit. You can ask us to audit just one process or multiple processes. For example, if you are the Quality Manager and the only qualified lead auditor in your company, you might want us to audit your internal auditing, CAPA, management review, control of documents, and control of records. For a single process audit, we generally recommend remote audits via Zoom in order to eliminate the cost of travel. This is also a great way to test us before you engage our firm for a full-quality system internal audit. This is also known as the “audit scope,” and should not be confused with “audit criteria” discussed below. The scope can also include the location of the audit. If you want us to conduct the audit remotely via
If you want us to conduct the audit remotely via  Please enter the date or dates that you want us to conduct your audit. You can also specify before a specific deadline (e.g. before June 30th). If you want us to conduct an audit of multiple processes remotely, it would help to know what dates and or times of day you would prefer. You can also enter a phone number and say “call me” next to the phone number. Then Lindsey or one of our assigned auditors will contact you to schedule a date and time for your audit.
Please enter the date or dates that you want us to conduct your audit. You can also specify before a specific deadline (e.g. before June 30th). If you want us to conduct an audit of multiple processes remotely, it would help to know what dates and or times of day you would prefer. You can also enter a phone number and say “call me” next to the phone number. Then Lindsey or one of our assigned auditors will contact you to schedule a date and time for your audit.  Please enter the desired duration of the auditing services you want to be quoted. We typically expect at least 30 minutes of audit preparation to review the audit preparation documents that you provide and to create an audit agenda. In addition, we expect to spend approximately two hours of report writing time for each eight-hour day of auditing. Therefore, a typically one-day supplier audit will require a duration of ten hours, while a three-day on-site internal audit will require a duration of 30 hours.
Please enter the desired duration of the auditing services you want to be quoted. We typically expect at least 30 minutes of audit preparation to review the audit preparation documents that you provide and to create an audit agenda. In addition, we expect to spend approximately two hours of report writing time for each eight-hour day of auditing. Therefore, a typically one-day supplier audit will require a duration of ten hours, while a three-day on-site internal audit will require a duration of 30 hours. It is important to specify the audit criteria for your auditing services quote, because otherwise, we might assign an auditor that does not have training on that criteria. Audit criteria are the standards, regulations, procedures, and contracts that may be used to evaluate your quality system or an individual process. Most of our audit team is qualified to audit against the following criteria:
It is important to specify the audit criteria for your auditing services quote, because otherwise, we might assign an auditor that does not have training on that criteria. Audit criteria are the standards, regulations, procedures, and contracts that may be used to evaluate your quality system or an individual process. Most of our audit team is qualified to audit against the following criteria: