Hiring an Auditor
In this article, you will learn how to hire an auditor to conduct medical device internal audits and supplier audits.

Hiring and Auditor
Hiring an auditor, whether as a consultant or a permanent team member, is a critical decision that can drastically improve your quality management system and foster a culture of quality, or it can add no value and lead to disruption and frustration. The purpose of this blog is to identify the qualities and training that make the best auditor to help you elevate your internal audit programs.
Audit Program Structures
Companies typically take one of the following approaches to address their internal audit requirements:
- Train internal personnel with other primary functions as auditors and have them audit other departments.
- Hire an independent 3rd party to conduct the internal audits.
- Build an internal audit team that is independent of all other processes.
Hiring an Auditor from Within
Option 1 is common across the industry and is a personnel-efficient means of achieving the audit objectives. While this type of approach can sometimes be effective and may satisfy the basic requirement to conduct internal audits, there can be some drawbacks to this structure. Sometimes, when people were not hired specifically to be auditors and auditing is something they were asked to do in addition to their regular job, there is little to no motivation to develop auditing skills, and the audits lack a depth and thoroughness that ultimately reduces the value of the audit program. Proper internal recruiting and training of these auditors is crucial to ensuring audits are a useful value-added exercise and not a box-checking chore.
To successfully recruit internal auditors serving in other roles, it’s important to motivate people to want to be an auditor. Let potential recruits know that employees with audit experience are more valuable to companies than those without. It exposes employees to upstream or downstream processes to better understand the overall operations and provides them the opportunity to make process improvements in both directions to their workflow. If you want to be effective and get promoted, you need to demonstrate value to your boss and top management. If you don’t understand what other departments need, how can you help them? No manager will promote a selfish, power-hungry hog. They promote team players that make others better. Auditing gives you the insight necessary to understand how you can do that.
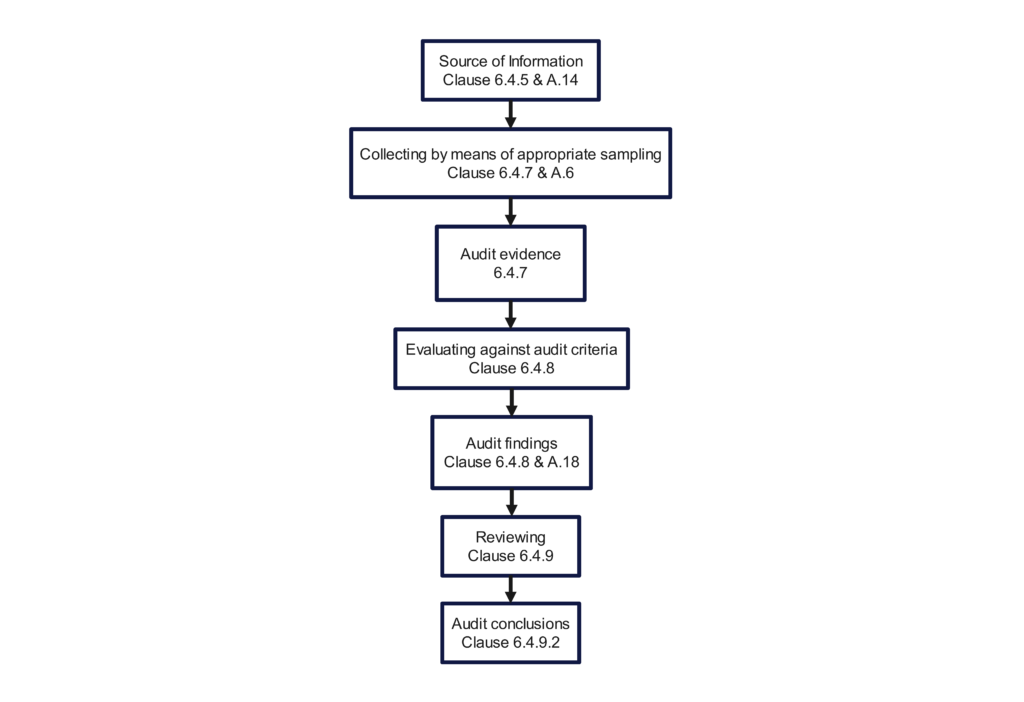
Once motivated and recruited, it’s important to ensure these employees have the skills and resources to be successful as auditors. To help develop their skills, training on audit processes and the responsibilities and role of an auditor in accordance with ISO 19011 will provide guidance on conducting audits and the basics of how to audit. Auditors should also be trained against the specific standard or regulation they are auditing against, which may include ISO 13485, 21 CFR 820, ISO 14971, EU MDR, and others. Resources that will support their activities may include process audit diagrams, checklists, examples of record requests, strategies for intelligent sample selection, and, of course, a clear definition of the regulatory and procedural requirements of the process that they are auditing.
If you are looking for support in training your own employees to be internal auditors, we would be happy to outline or provide a training program specific to your company’s processes and products to ensure your auditors are competent and effective in their new role.
Hiring a 3rd Party Auditor
Option 2 can be useful to any company, but selecting the right auditor is essential to the success of this approach. The basic qualifications and qualities that I recommend companies look for when hiring an outside auditor are:
- Experience – this includes industry experience and regulatory knowledge. An auditor with experience auditing or working for a company with similar devices, manufacturing processes, etc., will provide more value than an unfamiliar auditor. Regulatory knowledge and experience within your targeted markets are also important to evaluate to ensure that they are familiar with the standards and regulations against which they will be auditing.
- Communication Skills – This is a make-or-break quality of auditors that can shift the substance of an audit from a value-added exercise to a disrupting and frustrating experience. You want to ensure that auditors are affable yet confident, able to communicate the usefulness of the audit for the purpose of process improvement and facilitate a productive dialogue, offering education and suggestions when issues or nonconformances arise.
- Reputation and References – ask the auditor for references from previous clients. Contact the references to get feedback on their performance, reliability, and professionalism. This is a great way to evaluate an auditor’s communication skills and whether previous auditees gained value from the interaction.
- Auditor Training – acceptable qualifications for an auditor can be defined by the company but may include lead auditor certification, demonstrated training on relevant standards with experience shadowing experienced auditors, and documented training on other relevant standards/regulations.
- Audit Methodology – Inquire about how auditors plan, execute, and report on audits. What audit methodology does the auditor prefer for the scope of your audit, and why?
There are many companies and consultants that offer 3rd party auditor services, but not all are created equal. Like the CAPA process, the internal audit program is a window into the culture surrounding quality that your company has, and by demonstrating that you are proactively policing yourself and seeing continuous improvement through an effective internal audit program will show regulators that your company has a commitment to quality.
Hiring a Full-time Audit Team
Option 3 is generally reserved for the resource-rich industry with operations that demand expansive continuous audit processes to justify the support of a full-time auditor or audit team. Hiring your own team benefits from the same considerations that come with hiring a 3rd party auditor; the ability for the auditors to become intimately familiar with the company, devices, and processes is valuable. For companies that do not have the need for full-time auditors, the same value of familiarity can come from building a trusted relationship with a third-party auditor or audit team, who can support your audit program year after year.
Hiring an Auditor from Medical Device Academy
Our goal at Medical Device Academy is to help you improve your quality system and provide valuable consulting advice to achieve improvements. We specialize in helping start-up companies achieve initial ISO 13485 certification, MDSAP certification, and CE Certification. Based on the scope of the audit and medical device, we will assign the most qualified team member. Some of our specific areas of expertise include auditing companies with manufacturing and machining, aseptic processing, agile software development, sterile products, medical device reprocessors, 3D printed manufacturing, and more. If you are interested in outsourcing any supplier or internal audit activities, you can check out our Audit Services page to get in touch or to learn more about our audit team.