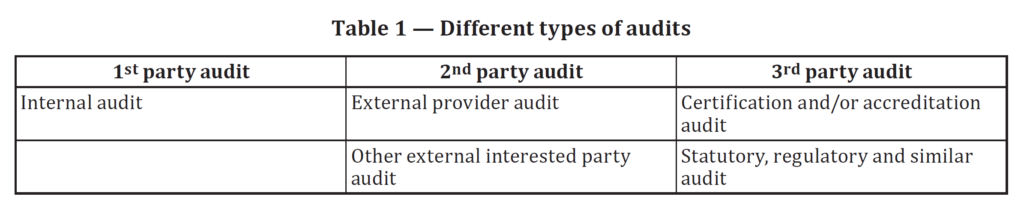
Nine easy ways to organize and improve quality system procedures
Would you like to learn nine ways to improve your quality system procedures? One method is precisely the opposite of our advice from 2011.
During a CAPA course I taught on Friday, January 28, 2011, one of the attendees asked if we teach a course on “How to write better quality system procedures.” Unfortunately, we could only offer material from a course about “Training the trainer.” That “Training the trainer” course focused on visual communication. Several books related to Lean Manufacturing explain how to use visual communication to replace text (i.e., “a picture says a thousand words”). During my ride home, however, I thought of a few other ideas that might help anyone writing or re-writing a procedure. The article was updated and posted as a new blog on Tuesday, March 28, 2023.
1. Use a standardized template for your procedures
In 2013 we published a blog about using a procedure template where we described our 12-part procedure template (i.e., TMP-001). You don’t have to mimic our template, but using a template will accelerate the speed of your writing when you create procedures, and it makes sure you don’t forget any of the essential elements. In addition, using templates ensures a consistent format that makes it easier for everyone to find the information they are looking for. Just make sure that your document control procedure allows flexibility to deviate from the template. The ISO 13485:2016 standard does require a “mandatory” format. Referring to your template as “suggested formatting” will avoid unnecessary nonconformities.
2. Create a process “turtle diagram” for each quality procedure
All of the procedures that Medical Device Academy created have a flow chart at the beginning of the procedure showing the procedures and forms associated with processes that are inputs to that procedure and outputs from that procedure. To systematically improve our procedures, we will be systematically replacing those flow charts with turtle diagrams for each process. This will give more detail than our current flow charts, and internal and external auditors can use the turtle diagrams to understand process interactions.
3. Avoid making unnecessary references to regulations and standards
If you are writing a procedure on risk management—it makes sense to reference ISO 14971. It does not make sense to reference all the other risk analysis standards unless you specifically use them to perform risk analysis. ISO 14971:2019, Clause 4.1, also states that you “shall establish, implement, document, and maintain an ongoing process for” risk management activities. However, the ISO 14971 standard is not directly linked to other procedures. Therefore, ISO 14971 should only be referenced in another if you are using it in that procedure or referencing it directly. For example, the Quality Manual (i.e., POL-001) explicitly references ISO 14971. In contrast, the design control procedure (i.e., SYS-008) references the risk management procedure (SYS-010) but doesn’t reference ISO 14971.
Concerning regulations, you should only reference regulations if the procedure meets a specific requirement. Color coding with symbols should demonstrate traceability to requirements (see method #5 below for further explanation). Rather than adding a reference to regulations in a procedure where there is no requirement, a better approach is to indicate in the Quality Manual that only procedures that have specific requirements will reference the regulations, such as 21 CFR 820 or Part 1 of the Canadian MDR.
4. Track standards, regulations, and the version used in your procedures
In the original 2011 version of this article, we advised quality managers to “avoid including the revision of a standard” because “this is just another opportunity for unnecessary nonconformities.” However, we find that our team has trouble identifying every procedure that a change in regulation or a standard might impact. A systematic process is needed to identify every procedure referencing a regulation or standard. Therefore, we will reference all impacted procedures next to the regulation or standard in our Master Document List (i.e., LST-001). References to the regulations will be added to the main tab for policies, procedures, and work instructions (i.e., [POL, SYS, and WI]). References to the standards will be added to the tab for documents of external origin (i.e., [Doc Ext Origin]).
Many people feel that you should not reference the version of a standard in a procedure because adding the version of the standard increases the number of documents that need to be updated when a standard changes. However, if you are only referencing standards in procedures when it is necessary, then that procedure should be reviewed and updated for the need to be changed. Updating the version of the Standard referenced is the best way to document that a gap analysis against the new version has been completed and the necessary updates were made to the procedure.
5. Use color coding and symbols in your quality system procedures

Matthew Walker, Medical Device Academy’s manager of the human factors team, has systematically updated many of our procedures to the EU Medical Device Regulations 2017/745 and the In Vitro Diagnostic Regulations 2017/746. When he updates our procedures, he references the regulations and applicable ISO 13485:2016 clauses. During certification audits, certification body auditors sometimes have difficulty finding where specific requirements are located in the procedures. Therefore, Matthew added color-coded clause references for our clients and auditors as a corrective action. To make the procedures inclusive for people that are color-blind, Matthew added symbols to supplement the color coding. The extra addition of symbols has proven invaluable because now anyone can search the documents electronically for a symbol to find where all the references are located.
6. Indicate the process owner and training requirements associated with each procedure
Identifying the process owner and training requirements in every procedure makes it easier to define who is responsible for reviewing and revising procedures. For the training requirements, the process owner should specify who needs to be trained on the process. Why? They know the procedure best. If there is a “grey area,” this should be resolved with the department manager for the job function. In addition, retraining requirements should be specified. The training section should also clarify if retraining is required when revising a procedure. If the revision is minor, training should only be necessary for people not trained on a previous revision.
7. Adopt the Plan-Do-Check-Act (PDCA) model for the structure of quality system procedures
For the “Plan” portion, the procedure should explain how to prepare to do something. This planning activity can apply to anything from planning to perform an audit to planning to inspect incoming raw materials. The “Do” portion is what most people refer to as the “Procedure” section. The “Check” portion of the procedure is a great place to specify the monitoring and measurement requirements for the process (see Section 8.1 of the Standard). Finally, the “Act” portion of the procedure should indicate what to do when target metrics are unmet. For example, what should be done when an alert limit is reached? What should be done when an action limit is reached?
8. Include the revision history of quality system procedures
It’s helpful to know which Document Change Notice (DCN) approved the document revision, why the changes were made, the nature of the changes, whether there is a related corrective action, and when the change was made. This will also tell auditors whether there is anything new to audit since the previous internal or external audit. This section is usually near the beginning of our procedures, but it doesn’t matter if the revision history is at the end or the beginning. However, it does help to be consistent.
9. Identify the form number, location, and retention period for each record
We have a section about quality system records near the end of every procedure. This section lists each quality system record that is associated with the procedure. The relevant form is referenced, but we recommend storing these records in electronic or paper folders labeled with the form number. If the files are digital, a hyperlink should be included. If the files are paper, then you should list the physical location of storage. The retention period can be listed in each procedure. Still, it will be essential to ensure that this information matches the regulatory requirements and record retention requirements in your “Control of Records” procedure (i.e., SYS-002).
Nine easy ways to organize and improve quality system procedures Read More »