The 3rd edition of the risk management standard for medical devices, ISO 14971:2019, was released on December 16, 2019.
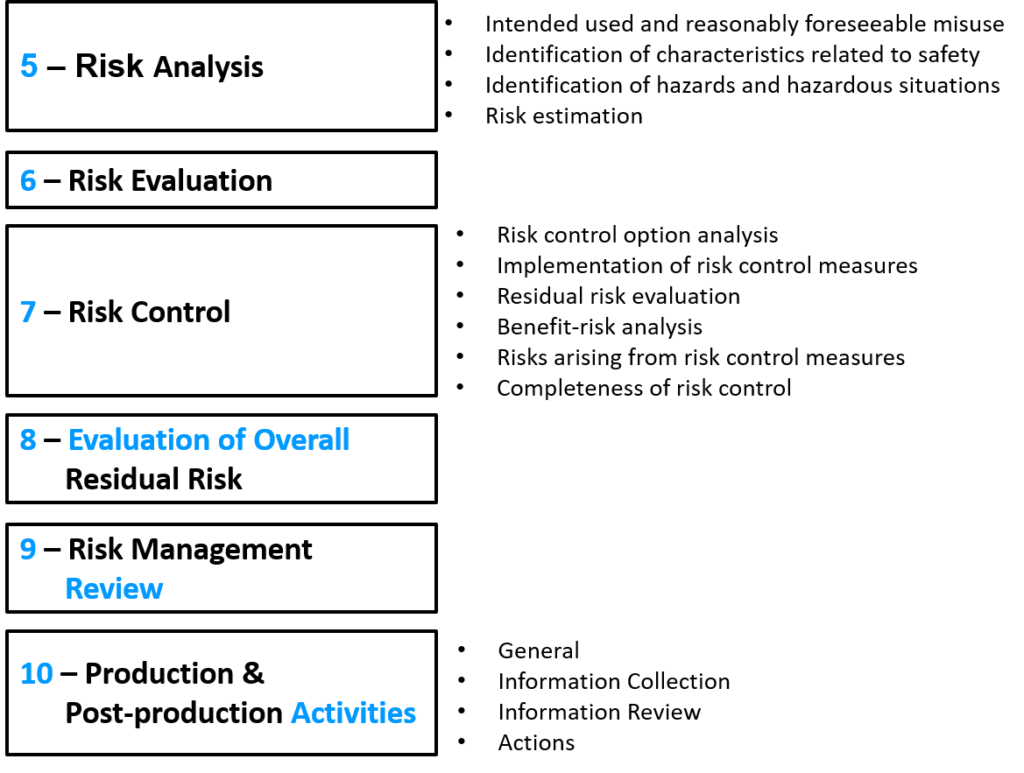
In October of 2018, I wrote a blog on the draft version of ISO 14971:2019 for risk management of medical devices. That article explained the differences between the different versions of the ISO 14971 standard (i.e., 2000, 2007, 2009, and 2012). I also explained what changed between ISO 14971:2007 and ISO/DIS 14971:2018. The final 2019 version of ISO 14971 3rd edition is now available.
The changes proposed in the draft included subtle changes to the names of the processes and a minor adjustment to the numbering of the clauses. Many of the annexes were also moved to ISO/TR 24971 guidance–which was released in 2020. The draft did not, however, result in a change in the overall process of risk management.
All of the changes that were discussed in my 2018 review were maintained in the final 2019 version that was released, but the ISO/TR 24971 guidance was not released at the same time as the committee had hoped for.
There are not any surprises in the 3rd edition (i.e., ISO 14971:2019). Therefore, I plan to wait until the ISO/TR 24971 guidance is released and then prepare a new blog specific to the guidance. If you are interested in training on the ISO 14971:2019 standard, the training I recorded on October 19, 2019, provides an excellent overview of these changes and highlights some of the challenges that you will encounter when trying to harmonize your risk management procedure between the ISO 14971:2019 standard and Regulation (EU) 2017/745.
Below are additional risk management resources:
- ISO 14971:2019 risk management training webinar – recorded October 2019
- Risk Management Procedure (SYS-010) that will be updated for the 2019 version (no cost for updates to our procedures)
- ISO/TR 24971:2020 risk management training webinar – February 10, 2020
This is a lot of information to absorb. Therefore, I recommend purchasing the October 2019 webinar and your copy of the ISO 14971 standard from AAMI. Anyone that has already purchased either the webinar or the procedure will receive an email offering them a discount on this new bundle that credits them for their previous purchase. If you have purchased both, you will receive credits for both purchases. Just think you can watch the video and read the new version of the standard while you are working out at the gym in January. Learn and burn!

Pingback: Nine easy ways to organize and improve quality system procedures Medical Device Academy
Pingback: Implementing Design Controls Medical Device Academy
Pingback: Iterative design is real, waterfalls are illusions Medical Device Academy
Pingback: Procedure template for ISO ISO 13485:2016 quality systems Medical Device Academy