This process approach webinar will teach you how to audit your quality system to ISO 13485:2016 or any other quality system standard.
When is the process approach webinar?
The process approach webinar was originally recorded on September 18, 2015. We are recording an updated webinar on June 23, 2020, @ 2:00 pm EDT. If you already purchased the webinar, you will receive an automated email inviting you to participate in the webinar. If this is a new purchase of the webinar, you will receive a link for downloading the old webinar and an invitation to participate in the new live webinar. Everyone that purchases the webinar will receive a link to download the recording if they are unable to attend the live webinar.
Changes to the process approach to auditing since September 18, 2015
When the previous version of this webinar was recorded, the final draft of the ISO 13485:2016 standard had not yet been released. In addition, the current version of the guidelines for quality system auditing was updated in 2018 (ISO 19011:2018). There were no changes to the requirements for internal quality audits in the 2016 standard, but the clause numbering shifted from Clause 8.2.2 to 8.2.4. This shift was necessary to accommodate the addition of Clause 8.2.2 for complaint handling and Clause 8.2.3 for reporting to regulatory authorities.
The changes to ISO 19011 were more significant. ISO 19011:2018 includes the addition of the risk-based approach to auditing as one of the seven principles of auditing. Three sections of the guidance were also expanded: 1) audit program management, 2) how to conduct an audit, and 3) generic competency requirements for auditors. There was also an expansion of the concept of virtual audits (i.e. remote auditing) in Annex A of the guidance. The expansion of Annex A also included guidance on other new auditing concepts: organizational context, leadership, commitment, compliance, and supply chain.
Description of the process approach webinar
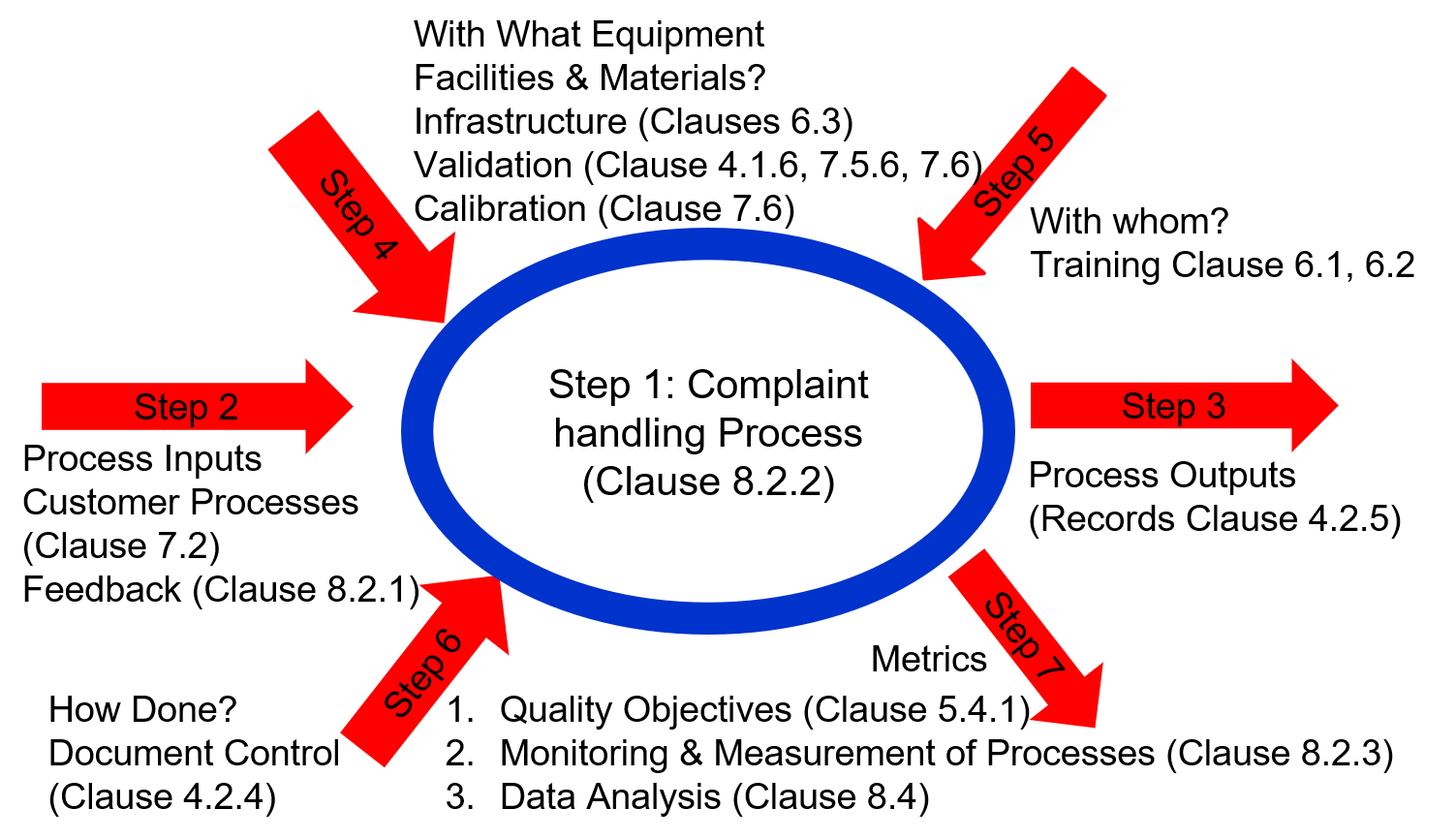
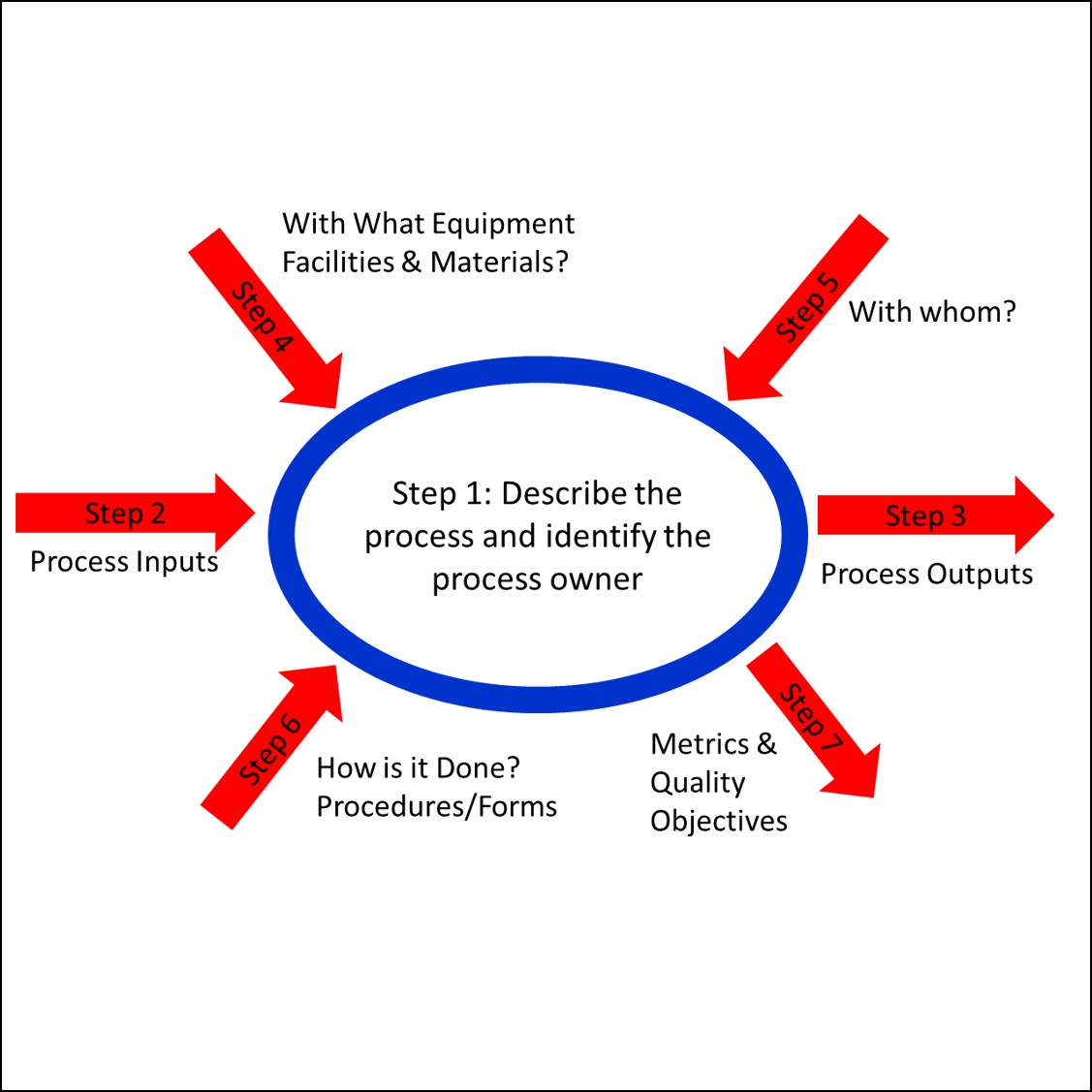
How to audit using the process approach webinar reviews how to effectively utilize the process approach to auditing. It also includes bonus materials. Internal auditors receive audit training and are generally told to use the process approach. Unfortunately, most auditors still use checklists organized by procedure or clauses of the ISO standard. However, checklists ignore process inputs and outputs and instead ask auditors to verify that a specific requirement of a procedure or the ISO standard has been met. Even though most auditors record objective evidence of the records sampled and the person interviewed, the checklist approach results in a superficial audit. Accredited lead auditor classes are required to teach the use of process turtle diagrams instead. Process turtle diagrams are a simple, seven-part tool used to take notes when you are auditing. All you need is a pen, a blank piece of paper, and training on how to use the process approach.
How to audit using the process approach webinar covers:
- What is the process approach to auditing?
- Why is the process approach more efficient than audit checklists?
- What is a turtle diagram?
- In what order should you ask audit questions?
- Who should you assign to each process and why?
- How you can add more value during audits?
WEBINAR OVERVIEW VIDEO:
Includes bonus material to keep in your educational toolbox
- Examples of 3 Turtle Diagrams Completed in Preparation for an Audit (PPT & Excel)
- Example Audit Notes Form for Design Process
- Example Process Audit Traceability Matrix
- Examples of 3 Audit Agendas Used in Presentation
- PLUS – Native PowerPoint Presentation included with this recording
Who should attend the process approach webinar?
- Supplier quality
- Quality assurance
- Auditors
- Lead auditors
- Audit program managers
- Senior management
All this for only $129.00
VIEW OUR PROCEDURES – CLICK HERE OR IMAGE BELOW:
About Your Instructor
 Robert Packard is a regulatory consultant with 30 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Robert was a senior manager at several medical device companies—including President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009-2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Robert’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510(k) submissions. The most favorite part of his job is training others. Specialties: CE Marking, Canadian Medical Device Applications, Post-Marketing Activities, Supplier Quality, CAPA, Risk Management, Auditing, Sterilization Validation, Lean Manufacturing, Silicone Chemistry, Extrusion, Bioprocess Engineering, and Strategy. He can be reached via phone at 802.258.1881 or by email. You can also follow him on Google+, LinkedIn, YouTube, or Twitter.
Robert Packard is a regulatory consultant with 30 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Robert was a senior manager at several medical device companies—including President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009-2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Robert’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510(k) submissions. The most favorite part of his job is training others. Specialties: CE Marking, Canadian Medical Device Applications, Post-Marketing Activities, Supplier Quality, CAPA, Risk Management, Auditing, Sterilization Validation, Lean Manufacturing, Silicone Chemistry, Extrusion, Bioprocess Engineering, and Strategy. He can be reached via phone at 802.258.1881 or by email. You can also follow him on Google+, LinkedIn, YouTube, or Twitter.