The video provided below shows you exactly what you will receive when you purchase our Software Validation Procedure (SYS-044).
The Software Development and Validation procedure aims to define the documentation requirements and the process for product software development and validation within the Company’s quality management system. The procedure is appropriate for companies developing software as a medical device (SaMD) and software in a medical device (SiMD).
Does the software validation procedure meet the most recent requirements?
This procedure is intended to meet the requirements of ISO 13485:2016, Clause 7.3.6 and 7.3.7 for design verification and design validation of medical device products. This procedure is also intended to meet the requirements of IEC 62304 and 21 CFR 820.30(a)(2)(i) and (g). The procedure content has also been updated to comply with the 2023 FDA guidance for software documentation in pre-market submissions. The 2023 guidance requires that companies document the software risk documentation level as basic or enhanced. TMP-019 can be used to document this and attached to the eSTAR in the section shown in the screen capture below:
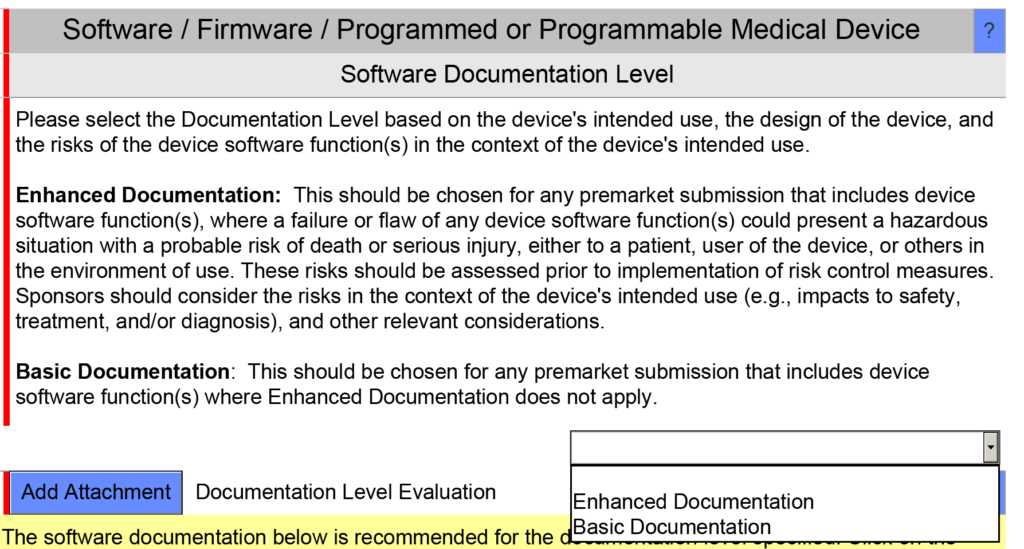
The FDA eSTAR also requires that companies attach nine different types of software validation documentation, as shown in the screen capture below:
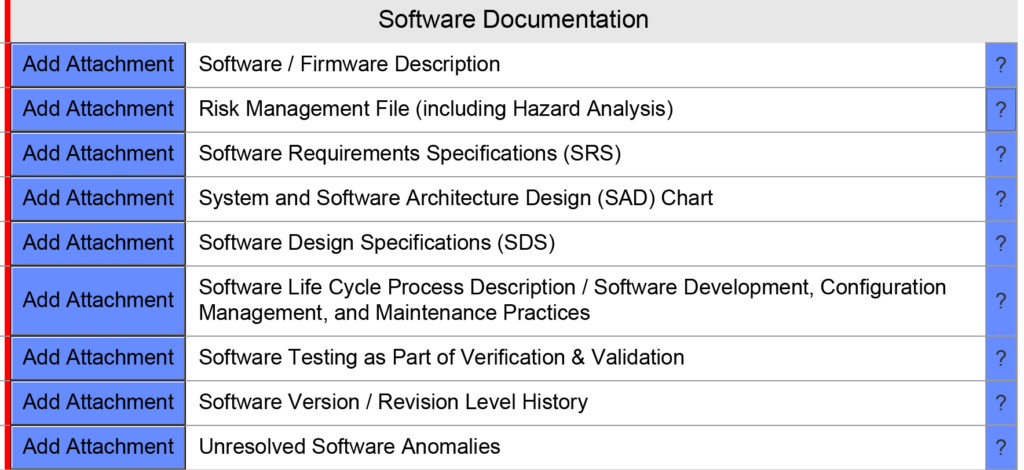
One of the less obvious changes in the requirements is a requirement for submission of a software risk management file. instead of providing only a software hazard analysis:
- A risk management plan,
- Risk assessment (hazard analysis), and
- Risk management report.

Does this software validation procedure include quality system software?
This procedure does not apply to the requirement for quality system software validation in ISO 13485:2016, Clause 4.1.6, validation of software for automated equipment in Clause 6.3 and 7.5.6, or validation of software used for monitoring and measuring calibrated equipment and devices in Clause 7.6. Those requirements are covered in SYS-014 Process Validation procedure, and the applicable technical standard is ISO 80002-2:2017.
This is the primary document meeting the applicable regulatory requirements for software development and validation as defined in the Quality System Manual (POL-001).
What is included with the purchase of this procedure?
The following is a list of documents included:
- SYS-044 A, Software Development and Validation
- TMP-009 Software Development Plan Template
- TMP-010 Software Requirements Specification Template
- TMP-011 Software Traceability Matrix Template
- TMP-012 Software Architecture Specification Template
- TMP-013 Software Design Specification Template
- TMP-014 Software Revision Level History Template
- TMP-017 Software Development Environment Description Template
- TMP-018 Software Description Template
- TMP-019 Documentation Level Evaluation Template
- TMP-020 Software Risk Management Plan (RMP)
- TMP-025 Verification Protocol Template
- TMP-026 Verification Report Template
- Native Slide Deck for Software Validation Webinar (March 7, 2024)
- Recording of Software Validation Webinar (March 7, 2024)
In addition to the procedures and templates, we have also created a zip folder with all four of the FDA guidance documents for software submissions:
- Guidance for the Content of Premarket Submissions for Software Contained in Medical Devices
- Guidance for Industry, FDA Reviewers, and Compliance on Off-The-Shelf Software Use in Medical Devices
- Guidance for Industry and Food and Drug Administration Staff Content of Premarket Submissions for Management of Cybersecurity in Medical Devices
- Guidance for Industry, FDA Reviewers, and Compliance on Postmarket Management of Cybersecurity in Medical Devices
You also need to purchase a copy of IEC 62304:2006 from your favorite standards issuer. Please note: This product will be delivered to the email address provided in the shopping cart transaction. After the transaction is verified, please check your email for the download. To view all available procedures, click here.
Please note: This product will be delivered to the email address provided in the shopping cart transaction. After the transaction is verified, please check your email for the download. To view all available procedures, click here.
About the Author
 Matthew came to us with a regulatory background that focused on OSHA and NFPA regulations when he was a Firefighter/EMT. Since we kidnapped him from his other career, he now works in Medical Device Quality Management Systems, Technical/Medical Writing, and is a Lead Auditor. Matthew has updated all of our procedures for He is currently a student in Champlain College’s Cyber Forensics and Digital Investigations program, and we are proud to say that he is also a member of both the Golden Keys and Phi Theta Kappa Honor Societies! Matthew participates as a member of our audit team and has a passion for risk management and human factors engineering. Always the mad scientist, Matthew pairs his professional life in regulatory affairs with hobbies in the culinary arts as he also holds a Butchers/Meat Cutters certificate from Vermont Technical College. He can be reached by email. You can also follow him on LinkedIn or YouTube.
Matthew came to us with a regulatory background that focused on OSHA and NFPA regulations when he was a Firefighter/EMT. Since we kidnapped him from his other career, he now works in Medical Device Quality Management Systems, Technical/Medical Writing, and is a Lead Auditor. Matthew has updated all of our procedures for He is currently a student in Champlain College’s Cyber Forensics and Digital Investigations program, and we are proud to say that he is also a member of both the Golden Keys and Phi Theta Kappa Honor Societies! Matthew participates as a member of our audit team and has a passion for risk management and human factors engineering. Always the mad scientist, Matthew pairs his professional life in regulatory affairs with hobbies in the culinary arts as he also holds a Butchers/Meat Cutters certificate from Vermont Technical College. He can be reached by email. You can also follow him on LinkedIn or YouTube.

