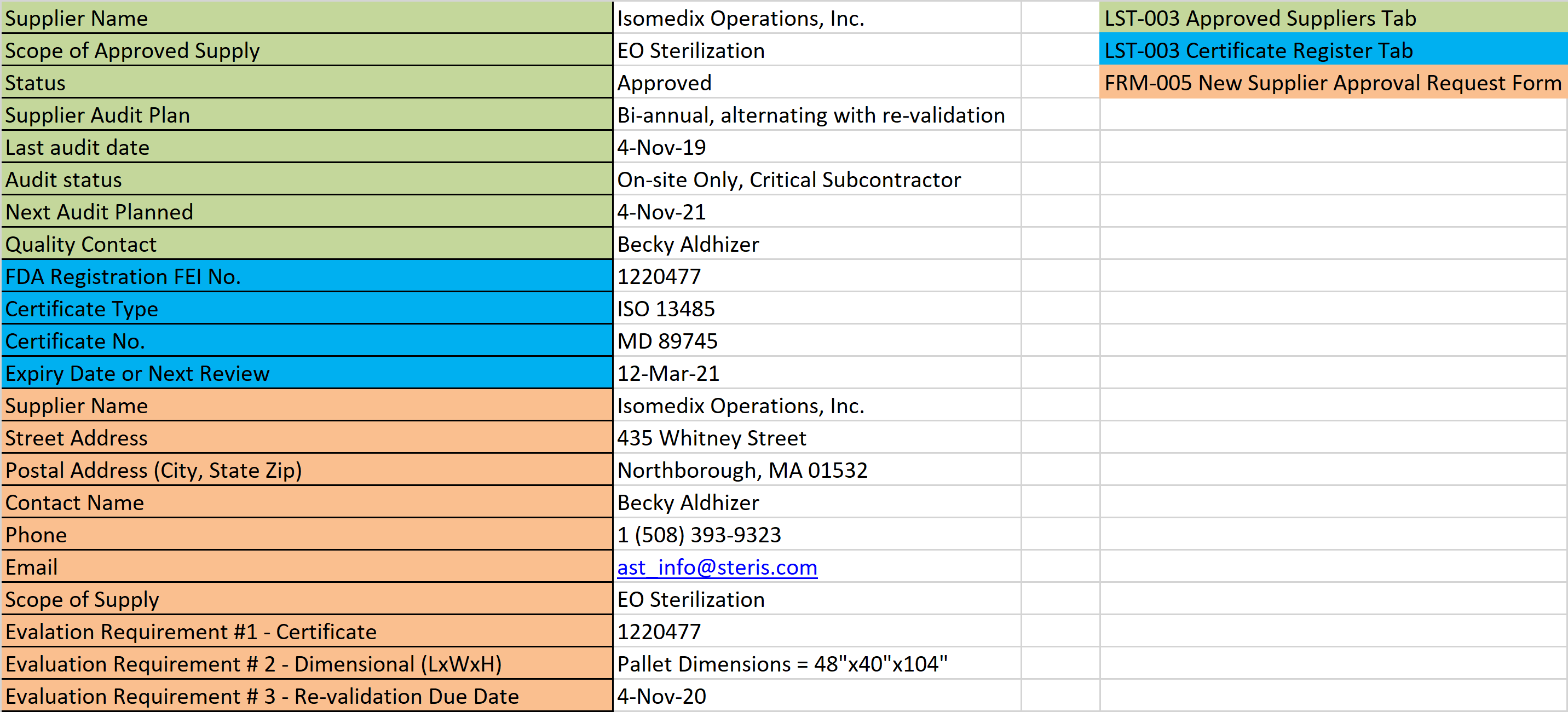
Device Supply Chain Disruptions
What can you do to stay ahead of medical device supply chain disruptions and comply with reporting requirements of possible device shortages?

Supply chain issues can be somewhat cyclical. As we approach the holiday season, we also approach the shipping season. Public shipping services such as FedEx and UPS see an increase in freight as the holiday seasons approach. Manufacturers need raw materials and components to stock the shelves with all of those holiday gifts. Since we are still living under pandemic conditions, I would be willing to bet there will be more care packages and mailed gifts in place of traditional gatherings. On top of the approaching increase in demand, staffing shortages can very quickly exacerbate supply chain bottlenecks. All the while importers are still expected to… well, import! If transportation affects all general industry you can bet it can also cause medical device supply chain disruptions.
So what does an overburdened mail service have to do with medical devices and quality systems?
Consider, how are your customers getting your product in their hands? How are you receiving raw materials and components? How about your contract manufacturer? Do they have supply chain redundancies? Does your supplier quality agreement address notifications for shipping disruptions?
Do you have a regulatory obligation to report a shortage/supply chain disruption or interruption of manufacturing to the FDA, or Health Canada? The FDA monitors for discontinuance and meaningful disruption of manufacturing certain devices and similarly Health Canada monitors their own list of devices for market shortages. Supply chain disruptions either through difficulty sourcing of raw materials and components, or through transportation breakdown of finished devices to market are just one way you could experience a reportable disruption or shortage.
Matthew did not choose the topic of medical device supply chain disruptions randomly. His signature brand of pessimistic cynicism is the reason we have him tasked with keeping his fingers on the pulse of global concerns and potential threats and risks. Potential supply chain disruptions will involve your quality staff in developing preventive actions and contingency plans in case there is an issue. Then, your regulatory team will be in charge of reporting and AHJ notification if you are an affected manufacturer (or importer in Canada!). Understaffed and overloaded shipping and transportation suppliers are about to be bombarded with seasonal freight. This makes them an attractive target for ransomware because, just like healthcare facilities, they will not be in a situation where they can afford any downtime.
Regulatory responsibilities related to device supply chain disruptions – Organized by Jurisdiction

The FDA requires reporting shortages and supply chain disruptions to CDHR of permanent discontinuance or interruption in manufacturing of a medical device in Section 506J of the FD&C Act. Especially so in response to the COVID-19 public health emergency. In part, the general public’s need for healthcare during the pandemic guides what devices the FDA needs notification about.
Currently, the FDA is concerned about specific device types by product code or any devices that are critical to public health during a public health emergency. For the most up to date list, the URL to the FDA website will show the specific product codes of the monitored device types;

As an Authority Having Jurisdiction, Health Canada also has reporting requirements for supply chain disruptions of specific types of medical devices. Health Canada is also an independent authority that uses a different device classification system than the U.S. FDA.
The table below shows the device types by their classification level that HC requires supply chain disruption notifications for. This information is current as of September 5th, 2021, and the following link will take you to the HC webpage for the most up-to-date list.
| Class I Medical Devices |
| Masks (surgical, procedure or medical masks) – Level 1, 2, 3 (ATSM) |
| N95 respirators for medical use |
| KN95 respirators for medical use |
| Face shields |
| Gowns (isolation or surgical gowns) – Level 2, 3 and 4 |
| Gowns (chemotherapy gowns) |
| Class II Medical Devices |
| Ventilators (including bi-level positive airway pressure or BiPAP machines, and continuous positive airway pressure or CPAP machines) |
| Infrared thermometers |
| Digital thermometers |
| Oxygen Concentrators |
| Pulse Oximeters (single measurement) |
| Aspirators/suction pumps (portable and stationary) |
| Laryngoscopes |
| Endotracheal tubes |
| Manual resuscitation bags (individually or part of a kit) |
| Medical Gloves – Examination and Surgical (Nitrile, Vinyl) |
| Oxygen Delivery Devices |
| Class III Medical Devices |
| Ventilators (including bi-level positive airway pressure or BiPAP machines) |
| Pulse Oximeters (continuous monitoring) |
| Vital Signs Monitors |
| Dialyzers |
| Infusion Pumps |
| Anesthesia Delivery Devices |
| Class IV Medical Devices |
| Extracorporeal Membrane Oxygenation (ECMO) Devices |
How to prevent device supply chain disruptions
Harden your supply chain with redundancies. Now is the time to qualify a second supplier as a contingency plan before it is too late…. Maybe even consider opening a Preventive Action? (HINT HINT for those ISO 13485 manufacturers that need to beef up their Clause 8.5.3. operations!)
Supply chains have both up and downstream functions. First, you likely need to source raw materials and components for production. Then you also need to ship those finished devices to distribution centers and your customers. Disrupt either of those and your ability to sell your devices is compromised or even completely halted.
Ask yourself, “Do I have a backup option for shipping?”, and “Do I have a backup option for raw materials and components?”.
Why?
Why go through all of that effort? Well, if you lose UPS and have to use FedEx instead, are their shipping procedures identical? Likely you will need a WI level document for each shipper to explain the process. It is easier to pre-qualify a contingency supplier and establish a WI now rather than in December when holiday shipping is at its peak. Consider if you also need to open accounts, etc. Scheduling pickup online may not be intuitive.
Just identifying a backup is important, but you can take that a step further and pre-qualify them. If they are a shipping and transportation supplier then give them a shipment or two in order to evaluate them. Hold them to the same standards you would for your primary supplier.
Did your shipment arrive on time? Was it damaged during transit? This is provisional, or pre-qualification. Did they perform adequately enough to use as a tentative supplier in the event the primary supplier is unable to perform? This is designed to make a full qualification of this supplier simple and easy… If you need to utilize them that is. Maintaining this pre-qualification should also be simple and easy as well. Once a year or so have them deliver a shipment for you.
That is just for importing or shipping finished devices. Do you have backup raw material or components suppliers identified? If not identifying or even pre-qualifying secondary suppliers might not be a bad idea either. You are probably tied down to a specific geographic area for shipping and transportation. You may not be for raw materials. If you need barrels of silicone consider a backup supplier from a different area than your primary supplier. Natural disasters create havoc for shipping. If your silicone comes from Company A, and they are closed down because of a hurricane then Company B ten miles away is likely affected as well.
For example, if you are in the U.S. and your primary supplier is in the Northeast then a backup supplier in the Southeast may be strategically important. Whereas a backup supplier from the Southwest may be cost-prohibitive.
What about your suppliers? Is your device high-risk enough that if your supply chain is disrupted, you have an obligation to report it to the FDA? In that scenario, if you use a contract manufacturer, it may be worth requiring supply chain contingencies and clearly identifying who owns what reporting responsibilities within your quality agreement with them.
There is an element of proactive responsibility in reporting these shortages, or projected shortages. In order to be able to predict medical device supply chain disruptions, there should be metrics that your quality system is monitoring. What is your monthly production capacity? How much raw material or components does your warehousing have on hand? How many units could you manufacture if the transport industry stopped right this second?
Determine what you need to track in order to identify a disruption before it occurs.
Prepare for notification now. This article looked at the problem from the point of view that transportation issues were the root cause of the supply chain disruption. However, many other things could be disruptive, such as natural disasters and supply availability. Therefore, develop a WI level document for conducting these types of regulatory reporting activities and train personnel before a disruption happens. It is easier to tackle these kinds of problems if you already have process controls in place and trained competent staff than if you wait until the reporting timeline clock is already ticking.
See also our blog article on Medical Device Shortage Reporting and Device Supply Chain Disruptions
Future blogs about device supply chain disruptions…Shortage Reporting
About the Author

Matthew Walker – QMS, Risk Management, Usability | Human Factors Engineering, Cybersecurity & DFIR
Matthew brings a unique background as a former Firefighter/EMT and Rope Rescue Tech with expereince in OSHA and NFPA regulations. For the better part of a decade, he has worked as a Technical/Medical Writer and Lead Auditor. He holds degrees in Fire Science and Computer Forensics and Digital Investigations, graduating Summa Cum Laude from Champlain College. Matthew is also an active member of several academic honor societies including Omicron Sigma Sigma’s Order of the Sword and Shield. His professional focus includes Human Factors Engineering, Risk Management, and Cybersecurity with a special interest in applying Digital Forensics and Incident Response (DFIR) practices to medical technology. He combines regulatory expertise with technical insige to strengthen both product safety and oranizational resiliance. He can be reached by email. You can also follow him on LinkedIn or YouTube.
Device Supply Chain Disruptions Read More »