Grant Writing – 5 common mistakes by medical device companies
Would you like to learn the five most common grant writing mistakes medical device companies make when seeking funding?
Why do most medical device companies fail grant writing?
Many medical device companies will face unique challenges in grant writing that can lead to failure. One common issue is a lack of alignment between the company’s objectives and the funding agency’s priorities, as grants often emphasize societal impact or public health benefits over market potential. Companies may also struggle with presenting their proposals effectively, using overly technical language that alienates reviewers or neglecting critical aspects like societal impact and feasibility. Limited resources, such as insufficient time, staff, or grant writing expertise, exacerbate the problem, as does the absence of strong collaborations with research institutions or public health organizations. Additionally, companies frequently underestimate development timelines, submit proposals prematurely, or fail to justify their budgets convincingly.
What are the most successful grants for device companies?
In the United States, prominent grant programs such as those offered by the NIH, NSF, and CDMRP play a significant role in supporting the design and development of medical devices. These agencies release funding opportunities multiple times a year, focusing on medical device innovations. The NIH allocates $1.3 billion annually to fund cutting-edge technologies. Similarly, the NSF receives approximately $190 million each year to support technological advancements across various industries, with a significant emphasis on medical devices, particularly those involving high-risk, high-reward technologies. Meanwhile, the CDMRP, through the Department of Defense, provides funding for medical devices with dual-use applications, targeting both military and commercial markets. These programs offer valuable resources for companies seeking to innovate in the medical device sector.
How often do grant applications get rejected?
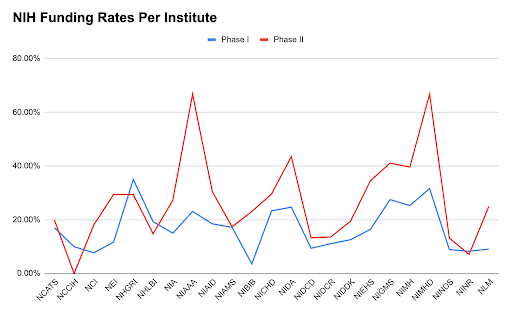
The path to success is highly competitive, with a substantial number of proposals facing rejection. Understanding the acceptance rates, common pitfalls, and strategies for improvement can enhance the likelihood of securing SBIR funding.
Acceptance rates for SBIR proposals vary across agencies and phases:
- Phase I: This initial phase focuses on establishing the technical merit and feasibility of the proposed research. Acceptance rates typically range from 10% to 20%, depending on the agency and specific solicitation. For instance, the National Institutes of Health (NIH) reported a success rate of approximately 25% for SBIR Phase I proposals in recent years.
- Phase II: Building upon Phase I results, Phase II emphasizes the development of the proposed innovation. Acceptance rates are generally higher, around 40% to 50%, as applicants have already demonstrated initial feasibility. NIH data indicates a success rate of about 44% for SBIR Phase II proposals.
Common Mistakes
The secret to successful grant writing is not just better writing. Timely preparation, ignoring program officer feedback, and matching your technology with an appropriate grant are just a few of the secrets to better grant writing.
Lack of Timely Grant Preparation
The SBIR agencies typically have a specified window every year of when the solicitation topics will be released. Common mistakes companies make is that they do not know when the announcements are made and where to find them. Many companies who miss the announcements fail to prepare in a timely manner to submit a winning grant proposal.
Not Seeking Feedback from Program Officers
During each solicitation cycle, each agency offers support from their program officers. These program officers make their contact information readily available to the public. Program officers are your best friend when you have any questions in regards to the application process and specific topics. Prior to submitting the proposal, you can meet with the program officers to ensure your project is the right fit for the topic you are seeking to apply for.
Not Matching the Technology with the Best-Suited Agency
There are 11 different agencies that partake in the SBIR program with varying focus areas. There is some overlap between these agencies and companies who want to pursue SBIR grants for the first time can miss a better suited opportunity from other agencies. It is important to take all things into consideration from funding amounts, solicitation topics, and award rates. There may be a specific topic from an agency that has a high award rate. However, if your project does not meet the needs for the topic and agency, it does not guarantee that you will be awarded. Hence why speaking to a program officer can guide you to your best-suited topic.
Not Enough Focus on the Business Side
As part of your SBIR proposal there is a heavy emphasis on commercialization, many for Phase II applications. There are lots of companies who have a great team with higher education, amazing scientific breakthroughs. However, they lack the expertise on how to bring this product to the market. A large miss on companies who do not get awarded is due to the inability to communicate in the proposal on how they will bring their product to the market. Through the process, you have to keep in mind that your business plans are just as important to your science.
Not Complying with Solicitation Requirements
At the times of when the solicitations are released, you have access to the documents on the requirements. This includes the format of the proposal, key information that is required and detailed information on the innovation the agency is seeking for the topic. The reason these documents are provided is to be of your resource. Failure to comply with these requirements is almost guaranteeing a loss. The first step to success is reading through the solicitation documents thoroughly and asking questions to experts.
What are the secrets to successful grant writing?
If your company has already won a phase 1 SBIR grant, and you are applying for a phase 2 SBIR, make sure that you obtain written feedback from the US FDA on your regulatory strategy in a pre-submission meeting request before your application deadline.
What can you do now to improve your chances of success?
Being awarded a SBIR award is never a guarantee but there are steps you can take to increase your likelihood of being awarded.
- If you have never written an SBIR proposal, seek expert guidance. There are lots of intricacies and changes that happen for the SBIR program and especially within the agencies themselves. There are many experts who are paid to do the dirty work all day for you! These experts will be able to understand your science and needs for your business. Therefore, eliminating headaches for your company to spend time researching on how to best write a grant proposal, leaving time to focus on your innovative technology that will change society.
- Start from Phase I. Many of the programs offer fast track programs to do a Phase I and Phase II proposal combined. As these are great opportunities to win larger grant funding amounts earlier on, your success is much lower. The fast track programs are a great opportunity if you can show you have the research completed that is needed when applying to a Phase I grant. Get your foot in the door through a Phase I award, win and then pursue a Phase II. This will increase your chances long term with the agency.
- Have a strong team. Your company does not need a large team but an experienced one. Those on your team must be fluent in your technology and business. Even if you are just an individual, you must have the background to prove your strong understanding of your technology and business.
- Do not stop trying! Most companies obtain success upon their second submission. Almost all agencies will provide scoring and/or feedback on your proposal. Use this feedback to enhance your next submissions increasing your likelihood of success.
Grant writing resources
About the Author
 As the Marketing Lead at BW&CO, Alexina holds a Bachelor of Business Administration in Marketing from the University of Houston. With a deep understanding of the grant funding landscape, she drives strategic marketing initiatives that elevate BW&CO’s brand and reach. Fluent in both English and French, Alexina excels at creating and executing campaigns that connect with diverse audiences. Her expertise includes developing content strategies, managing digital marketing efforts, and conducting market research to support business growth.
As the Marketing Lead at BW&CO, Alexina holds a Bachelor of Business Administration in Marketing from the University of Houston. With a deep understanding of the grant funding landscape, she drives strategic marketing initiatives that elevate BW&CO’s brand and reach. Fluent in both English and French, Alexina excels at creating and executing campaigns that connect with diverse audiences. Her expertise includes developing content strategies, managing digital marketing efforts, and conducting market research to support business growth.
+1 (774) 257-8760
Grant Writing – 5 common mistakes by medical device companies Read More »
