How to select and help validate the best sterilization method?
The FDA eSTAR includes a list of eight different options for a sterilization method, but how do you select the best method and validate it?
What is Sterile Packaging Day?
The Sterilization Packaging Manufacturers Council (SPMC) founded Sterile Packaging Day in 2021 to recognize and thank all of the companies in the supply chain who work together to deliver innovative, safe, and sterilized devices to provide excellence in patient care. Sterile Packaging Day is February 8, 2023, and this year’s celebration theme is “Designed to Protect.” SPMC provides four tips for celebrating Sterile Packaging Day:
- Donate blood (use this link for an appointment) on February 8, 2023, at MD&M West in Anaheim
- Recognize and thank an esteemed packaging professional with whom you collaborate for success
- Support the next generation of packaging engineers (FPA Student Design Challenge)
- Tell us in one word what “Designed to Protect” means to you (Rob chose “Lifesaving”)
Thank you to Jan Gates!
How to select the best sterilization method
Several factors determine the best sterilization method to use for your device. The first factor is whether your device will be delivered sterile or will the end user sterilize the device. If the end user is responsible for sterilizing the device, the most common methods used by hospitals are:
- steam sterilization
- hydrogen peroxide sterilization
- EO sterilization
The popularity of the third method is declining due to environmental restrictions on hazardous emissions from the ethylene oxide sterilization process. Hydrogen peroxide is gaining popularity because it can be used for heat-sensitive materials, and hydrogen peroxide vapor reacts with moisture to form a harmless aqueous solution. Steam is the most common sterilization method used by doctors, dentists, and hospitals because steam sterilizers are relatively inexpensive, and no hazardous chemicals are required.
The second factor to consider when selecting a sterilization method is whether there are any heat-sensitive components. Plastics will melt and degrade in dry heat sterilization cycles, and some plastics cannot withstand the temperature of a steam sterilizer. Therefore, if your device is constructed from plastics for cost reduction, weight, magnetic resonance (MR) compatibility, or other reasons, you may need to use a sterilization method with a lower temperature process.
The third factor to consider when selecting a sterilization method is whether any long, narrow tubes require sterilization. These design features are difficult to sterilize for any vapor-based sterilization process, such as steam, hydrogen peroxide, or ethylene oxide. There are a few process control strategies that can be used to sterilize with gas:
- use of an extreme vacuum to improve penetration of sterilant gas
- ensuring that the device and packaging materials are dry
- use of longer cycles with more sterilant gas
- use of internal biological indicators at the most difficult sterilization location
The fourth factor to consider when selecting a sterilization method is whether the device includes a liquid. A liquid cannot be sterilized with hydrogen peroxide, ethylene oxide, or dry heat. In some cases, the liquid may be a sterilant (i.e., ISO 14160:2021 for liquid chemical sterilizing agents). There are three popular solutions for the sterilization of a device that includes liquid:
- steam sterilization–assuming the liquid doesn’t contain components that are heat sensitive (e.g., proteins)
- filter sterilization–usually combined with aseptic filling and pre-sterilizing containers)
- radiation sterilization with eBeam or Gamma
eBeam and Gamma are also used for sterilizing products where cross-linkage of ultra-high molecular weight polyethylene (UHMWPE) is desired, or it is impossible for a gas sterilant to penetrate all areas of a device.
What are the applicable sterilization validation standards for each sterilization method?
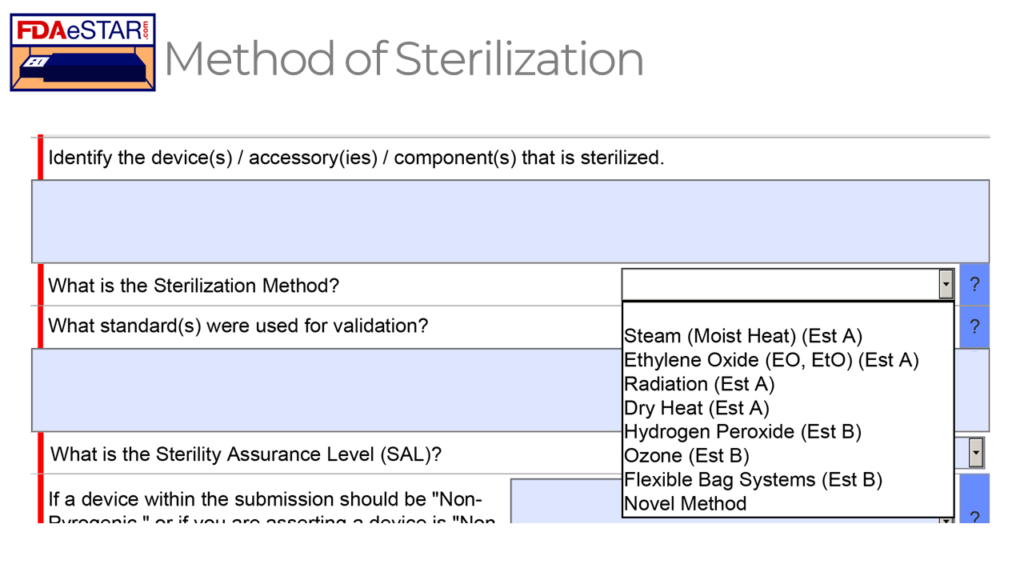
As shown in the FDA eSTAR screen capture above, eight possible sterilization methods can be selected for sterilizing a medical device in a 510k or De Novo submission. Each sterilization method has a different applicable standard that should be used to validate the sterilization process, but in all cases, the sterilization process must result in a sterility assurance level (SAL) of 10-6.
The FDA feels that the Established A (Est A) methods of sterilization have a long history of safe and effective use, while the FDA has not recognized a dedicated consensus standard for the Established B (Est B) sterilization methods. However, there are examples of devices that have received FDA 510k clearance using each of the non-traditional sterilization methods (i.e., Est B methods). Manufacturers will generally adapt existing international standards for sterilization validation to validate the non-traditional methods. There is published information on the development, validation, and routine control for these non-traditional sterilization processes.
Links to each of the recognized standards are provided below:
- Steam (Moist Heat) (Est A) – ISO 17665-1:2006, Sterilization of health care products — Moist heat — Part 1: Requirements for the development, validation, and routine control of a sterilization process for medical devices
- Ethylene Oxide (EO, EtO) (Est A) – ISO 11135:2014, Sterilization of health care products – Ethylene oxide – Requirements for development, validation and routine control of a sterilization process for medical devices; and ISO 10993-7:2008, Biological evaluation of medical devices – Part 7: Ethylene oxide sterilization residuals
- Radiation (Est A) – ISO 11137-1:2006, Sterilization of health care products – Radiation – Part 1: Requirements for development, validation, and routine control of a sterilization process for medical devices; ISO 11137-2:2013, Sterilization of health care products – Radiation – Part 2: Establishing the sterilization dose
- Dry Heat (Est A) – ISO 20857:2010, Sterilization of health care products – Dry heat – Requirements for the development, validation and routine control of a sterilization process for medical devices
- Hydrogen Peroxide (Est B) – ISO 22441:2022, Sterilization of health care products — Low temperature vaporized hydrogen peroxide — Requirements for the development, validation and routine control of a sterilization process for medical devices (this standard is not recognized by the US FDA)
- Ozone (Est B) – this is a new method using Ozone gas, and the method of action is similar to EO and H2O2
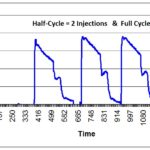
- Flexible Bag Systems (Est B) – ISO 22441:2022 should be used for validation of flexible bag systems with hydrogen peroxide, but instead of validating the process with three half-cycles that are half the duration of the full-cycle, instead, you use three half-cycles that use half the volume of sterilant of a full-cycle; this method is used by Andersen Scientific for their EO Bag sterilizers.
- Novel Methods – ISO 14937:2009, Sterilization of health care products – General requirements for characterization of a sterilizing agent and the development, validation and routine control of a sterilization process for medical devices
When should you use a novel sterilization method?
Novel sterilization methods should only be used when none of the traditional (Est A) and non-traditional (Est B) sterilization methods will not work. For example, aseptic filling combined with filtration of liquids is a common strategy for pre-filled syringes if the liquid is sensitive to radiation sterilization. Sterilization with peracetic acid has been used for a long time, but the sterilization method has not gained widespread popularity. Peracetic acid can also be combined with hydrogen peroxide. There is also a low-temperature steam and formaldehyde validation standard (i.e., ISO 25424:2019). Sterilization with UV light is a process that is sometimes used where materials are sensitive to high temperatures and where all surfaces can be penetrated with UV light. Nitrogen dioxide was developed as a more environmentally friendly sterilant similar to ethylene oxide. X-Ray is a new type of radiation sterilization that is being developed as a high-speed alternative to Gamma and eBeam, but X-Ray sterilization also has the advantage of being able to control a narrower dose range than Gamma and eBeam processes.
Consensus Standards for Sterilization Validation
There are also additional supporting standards that you will need for validation of your sterilization process. The following is a partial list of the standards you might consider:
- ISO 11737-1:2018, Bioburden Testing for Aerobic Bacteria and Fungi
- USP<51> Antimicrobial Effectiveness Test
- Candida albicans (a yeast…yeasts are a form of fungus)
- Aspergillus brasiliensis (a filamentous mold…also a fungus)
- Escherichia coli (a bacterium…better known as “E. coli”)
- Pseudomonas aeruginosa (a bacterium….very problematic industrially)
- Staphylococcus aureus (a bacterium…better known as “Staph”
- USP<61> Bioburden or Microbial Limits Test (Total Aerobic Microbial Count = TAMC; Total Yeast and Mold Count = TYMC)
- USP<62> Objectionable Organisms or Pathogens Tests
- USP<63> Mycoplasma Tests
- USP<71> Bacteriostasis/Fungistasis (i.e., B/F) Sterility Tests
- ISO 11138-1:2017, Sterilization of health care products – Biological Indicators – Part 1: General Requirements
- ISO 111140-5:2017, Sterilization of health care products – Chemical indicators – Part 5: Class 2 indicators for Bowie and Dick air removal test sheets and packs
- ISO 17664-1:2021, Processing of health care products – Information to be provided by the medical device manufacturer for the processing of medical devices – Part 1: Critical and semi-critical medical devices
Aging and Shelf-life Testing
The current standard for accelerated aging studies is ASTM F1980:2021 “Standard Guide for Accelerated Aging of Sterile Barrier Systems and Medical Devices has been revised and recently released to include medical devices.” Jan Gates explains that the “and” used to say “for.” The language was updated with more information on product humidity effects to go with the title. Jan was kind enough to write a Shelf-life Testing Protocol for us based on this new version of the standard. The protocol includes requirements for real-time and accelerated age testing of a product. If you need basic training on how to validate the shelf-life of your device, we have a webinar for sale on sterility and shelf-life. We also recorded an updated webinar on January 19, 2023, as part of the FDA eSTAR updates to our 510(k) Course.
Distribution Conditioning Tests & Packaging Performance Tests
There are also standards for distribution conditioning tests (i.e., ASTM D4169-22). Jan Gates was kind enough to write a 20-page Distribution Conditioning Shipping Qualification Protocol for Medical Device Academy based upon the ASTM standard. The protocol is available for purchase at the link above. Jan also wrote an 18-page Packaging Performance Testing Protocol for our customers in accordance with ISO 11607-1 and ISO 11607-2.
Where can you find a procedure for each sterilization method?
ISO 13485:2016, Clause 7.5.7 is specific to the “Particular requirements for validation of processes for sterilization and sterile barrier systems.” This clause includes the requirement to establish procedures for sterilization validation and validation of your sterile barrier systems. Even if your company uses a protocol and procedures established by a contract manufacturer, you still need to establish an internal procedure(s) to meet this requirement if you have sterile products. The following is a list of procedures sold by Medical Device Academy:
- SYS-014 Process Validation (6 pages)
- SYS-031 EO Sterilization Validation (4 pages)
- EO Sterilization Protocol (19 pages)
- SYS-043 Steam Sterilization Validation Procedure (10 pages)
- SYS-046 Packaging Validation Procedure (4 pages)
- SYS-047 GAMMA Sterilization Validation Procedure (4 pages)
What is the process flow for contract sterilization?
Most device manufacturers do not sterilize their devices in-house. Instead, sterilization is outsourced to a contract sterilizer. The process flow diagram below is a hypothetical process flow diagram for a contract sterilization process. The only step not included in this process flow is the incubation of biological indicators because gamma and eBeam sterilization processes use dosimeters instead of biological indicators. The nature of biological indicators is also changing rapidly because manufacturers are developing “rapid test” biological indicators. In 2008 I worked extensively with self-contained biological indicators that eliminated the need to use an aseptic technique to transfer biological indicators into culture media. In addition, I complete an incubation reduction study to validate a shorter 48-hour incubation cycle instead of the typical 7-day sterility test. Terragene is one of the manufacturers developing next-generation technology for biological indicators that allows the results to be read within seconds instead of 48 hours. This next-generation technology also incorporates barcode readers and networked readers to ensure traceability to each biological indicator and reader.
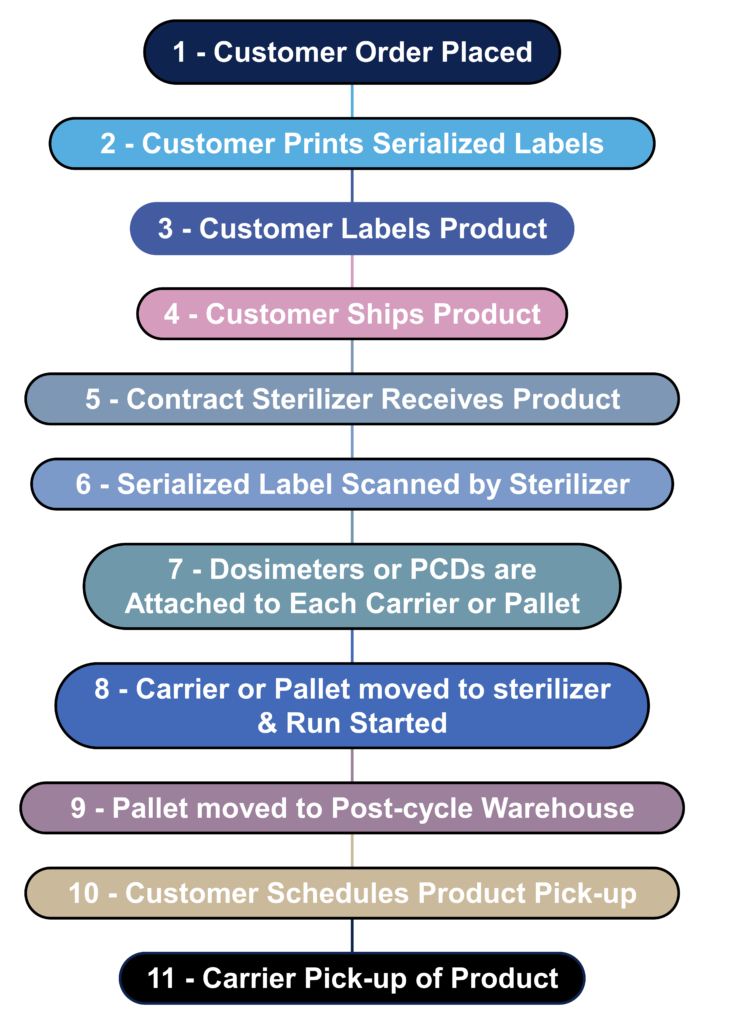
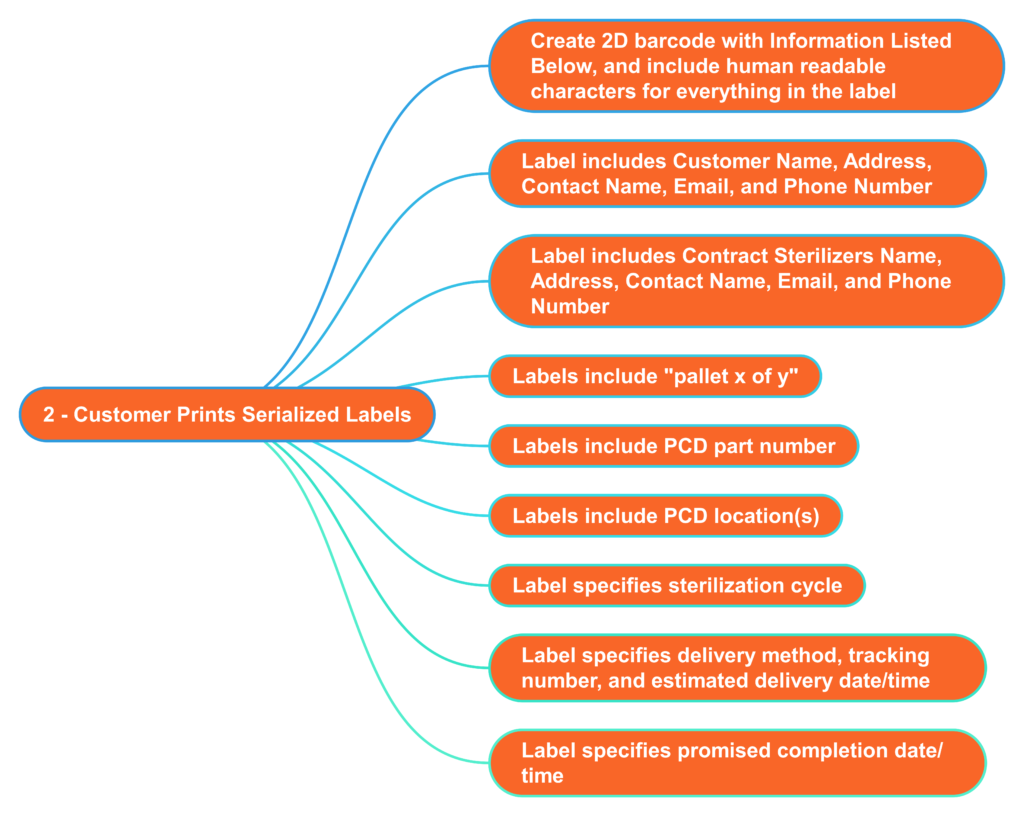
What information should serialized labels include for contract sterilizers?
In the “olden days” (c. 2005), I used to print out labels for each pallet that we shipped to the Isomedix facility in Northboro, MA. The label identified who the product was from and what we wanted Isomedix to do with the product (e.g., gamma sterilize at 25-40 kGy). At the time, we were just beginning to incorporate barcodes into on-demand labeling to facilitate traceability. 18 years later, companies are still stalling the implementation of on-demand barcoded labels. Almost every shipping dock has a barcode reader, and the technology is inexpensive. Therefore, you should consider creating a template for on-demand barcoded labels with all the information listed below. This will reduce the risk of errors by the contract sterilizer and enable you to identify when a mistake was made quickly. Contract sterilizers should also demand this information on product labeling as an added risk control. All biological indicators and dosimeters are labeled with UDI barcodes now. Therefore, contract sterilizers should be able to create robust process controls that ensure traceability between barcodes on your labeled product with barcodes on the biological indicator or dosimeter.
How to select and help validate the best sterilization method? Read More »