Packaging Complaint Investigation – Case Study
This is part one of a case study on how to perform a packaging complaint investigation when packaging is found open by a customer.
Overview of Packaging Complaint Investigation
This case study example involves a flexible, peelable pouch made of Tyvek and a clear plastic film. This is one of the most common types of packaging used for sterile medical devices. In parallel with the complaint investigation, containment measures and corrections are implemented immediately to prevent the complaint from becoming a more widespread problem. The investigation process utilizes a “Fishbone Diagram” to identify the root cause of the packaging malfunction. This is just one of several root cause analysis tools that you can use for complaint investigations, but it works particularly well for examples where something has gone wrong in production process controls, but we are not sure which process control has failed.
Description of the packaging malfunction
The first step of the complaint handling process (see SYS-018, Customer Feedback and Complaint Handling) is to record a description of the alleged quality issue. A distributor reported the incident that was reported. The distributor told customer service that two pouches in a box containing 24 sterile devices were found to have a seal that appeared to be delaminating. Unfortunately, the distributor was unable to provide a sample of the delaminated pouches or the lot number of the units. Packaging issues and labeling issues are typically two of the most common complaint categories for medical devices. Often the labeling issues are operator errors or a result of labeling mixups, while the packaging errors may be due to customers who accidentally ordered or opened the wrong size of the product. Therefore they may complain about packaging when there is nothing wrong. It is essential to be diligent in the investigation of each packaging complaint because if there is a legitimate packaging quality issue, then there may be a need for a product recall as part of your corrective action plan.
Initiation of the packaging complaint investigation
In your complaint record, you need to assign a person to investigate the complaint. The only acceptable reason for not initiating an investigation is when a similar incident was already investigated for another device in the same lot or a related lot (i.e., packaging raw material lot is the same and the problem is related to the material). If the complaint was already investigated, then the complaint record should cross-reference the previous complaint record.
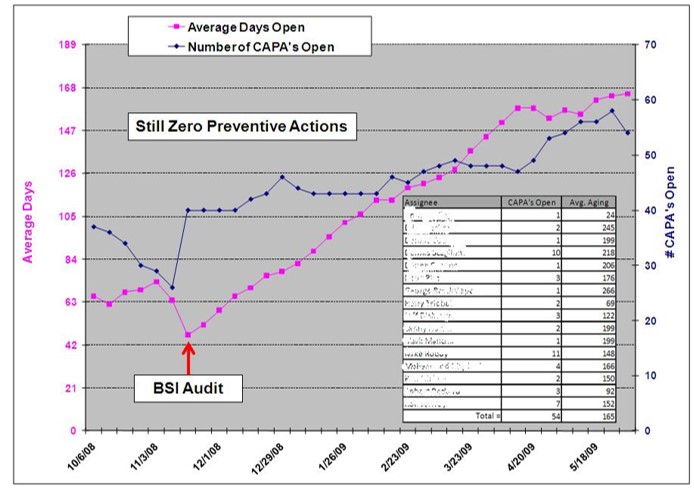
The person assigned to investigate the complaint must be trained in complaint investigations and should be technically qualified to investigate the processes related to the complaint (e.g., packaging process validation). The investigator must record which records were reviewed as part of the investigation, and the investigation should be completed promptly in case regulatory reporting is required or remedial actions are needed. It is also necessary to demonstrate that complaints are processed in a consistent and timely manner (e.g., average days to complaint closure may be a quality objective).
Regulatory reporting of packaging failures
We know everyone wants to avoid regulatory reporting because we are afraid that other customers will lose confidence in our product and bad publicity may impact sales. However, the consequences of failing to file medical device reports with the FDA are much worse. Even if an injury or death did not occur with a sterile medical device, the quality issue should still be reported as an MDR under 21 CFR 803 (see SYS-029, Medical Device Reporting) because a repeat incident could cause an infection that could result in sepsis and death. If you think that this is an extremely conservative approach, you might be surprised to learn that 251 MDRs were reported to the FDA in Q4 of 2023 for packaging issues. Of these reports, only one involved an actual injury, and the other 250 involved a device malfunction but no death or injury. The following event description and manufacturer’s narrative is an example:
Event Description
“It was reported by the sales rep in japan that during an unspecified surgical procedure on (b)(6) 2023 the rgdloop adjustable stnd device sterile package was not sealed and was unclean.Another like device was used to complete the procedure.There was an unknown delay in the procedure reported.There were no adverse patient consequences reported.No additional information was provided.”
Manufacturers Narrative
“This report is being submitted in pursuant to the provisions of 21 cfr, part 803.This report may be based on information which has not been able to investigate or verify prior to the required reporting date.This report does not reflect a conclusion by mitek or its employees that the report constitutes an admission that the device, mitek, or its employees caused or contributed to the potential event described in this report.If information is obtained that was not available for the initial medwatch, a follow-up medwatch will be filed as appropriate.Device was used for treatment, not diagnosis.If information is obtained that was not available for the initial medwatch, a follow-up medwatch will be filed as appropriate.H10 additional narrative: e3: reporter is a j&j sales representative.H4: the device manufacture date is unknown.Udi: (b)(4).”
Packaging complaint investigation when product IS NOT returned
What the narrative above does not elaborate on is what was the specific investigation details for “lot history reviewed.” One of the most useful tools for performing a packaging complaint investigation is the “Fishbone Diagram.” Other names include, “Ishikawa Diagram” and “Cause and Effect Diagram.” There are six parts (i.e., “6Ms”) to the diagram:
- materials,
- method,
- machine,
- “mother nature” or environment,
- “manpower” or people, and
- measurement.
What records can be investigated without the return of the product?
The following records could be reviewed and evaluated for potential root causes even if the customer does not return the packaging with the alleged malfunction:
- review the complaint log for other complaints with the same lot number and/or from a similar period, lot of raw materials, or packaging machine
- review the device history record for the lot to make sure that the number of units rejected as part of normal in-process and final inspection did not exceed pre-established thresholds for monitoring the sealing process
- if retains of the lot are available, these might be retested to verify that the testing results after real-time aging remain acceptable
- the maintenance and calibration records of the equipment for manufacture and testing may be reviewed to verify that no repairs were required and no equipment was identified as out-of-calibration
If all of the above fail to identify a potential cause for a packaging failure, then you might have a problem related to people or the environment. People include the people sealing the product package and the users. The environment consists of the temperature and humidity for storage of packaging raw materials, packaged products, sterilization conditions, storage conditions after sterilization, and shipping conditions–including any temporary extremes that might occur during transit.
In our case study, the product was not returned, and we did not have the lot numbers. Therefore, we may need to review distribution records to that distributor and/or the customer to narrow down the possible lots to one or more lots. Then we would need to perform the same type of review of lot history records for each potential lot. The best approach is to request a photo of the package labeling, including the UDI bar code, because that information will facilitate lot identification. Even if the product was discarded, often the UDI will be scanned into the patient’s electronic medical record (EMR) during surgery.
Conducting investigations when product IS returned
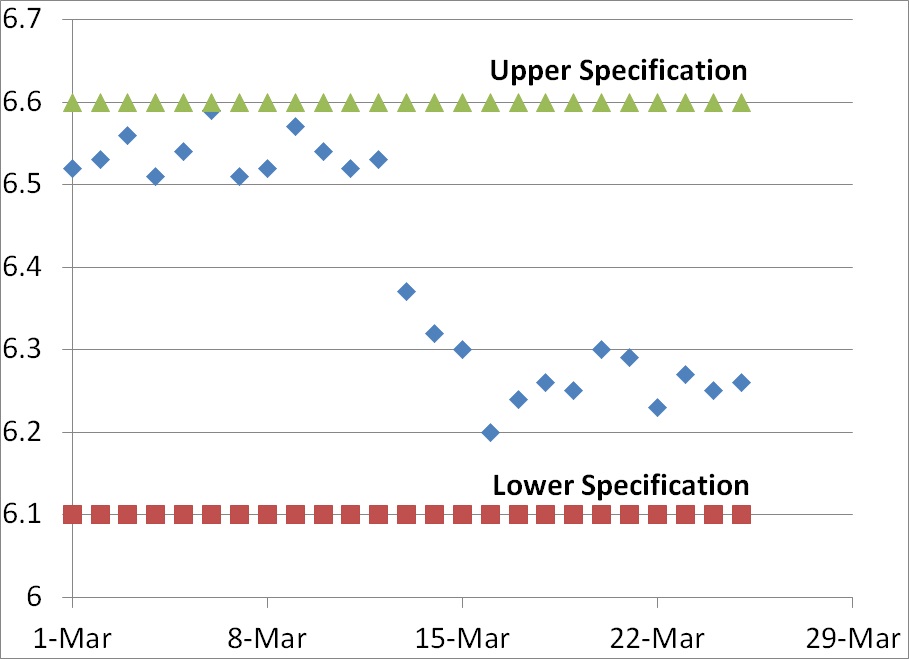
Sometimes you are fortunate enough to receive returned products. The product should be immediately segregated from your other products to prevent mixups and/or contamination. Normally the returned products are identified as non-conforming products and quarantined. After the quarantined product is evaluated for safety, the assigned investigator may inspect the packaging in a segregated area. Packaging investigations begin with visual inspection following ASTM F1886. If multiple packaging samples are available, or the packaging is large enough, the investigator may destructively test (i.e., ASTM F88) a 1” strip cut from the packaging seal to verify that the returned packaging meets the original specifications. If you kept retains of packaging with the same lot of flexible packaging, you may visually inspect and destructively test retains as well.
Next steps of the packaging complaint investigation
Once the root cause is identified for a packaging complaint, then you need to implement corrective actions to prevent a recurrence. Also, FDA Clause 21 CFR 820.100 and ISO 13485, Clause 8.5.3, require that you implement preventive actions to detect situations that might result in a potential packaging failure in the future and implement preventive measures so that similar packaging failures are not able to occur. If you are interested in learning more about conducting a root cause analysis, please read our blog on this topic: Effective Root Cause Analysis – Learn 4 Tools.
This article is the first half of the packaging complaint investigation case study. The second half of the two-part case study explains the necessary containment measures, corrections, corrective actions, and preventive actions to address the root cause of the packaging failure.
Additional packaging validation resources
There are many articles on the topic of package testing and package design for sterile medical devices. If you want to learn more, please register for our free webinar on packaging validation by Jan Gates.
Packaging Complaint Investigation – Case Study Read More »