Risk Control Options for Medical Devices: Deviation #6
This blog discusses risk control options for medical devices; the 6th deviation identified in the European National version of the Risk Management Standard.
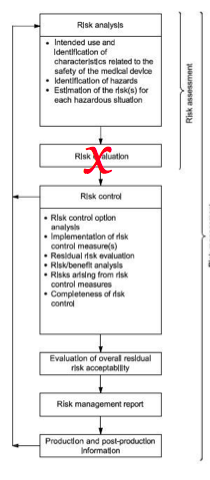
Design is not the same as design and construction. This is the interpretation of the European Commission. The sixth of the seven deviations identified in the European National (EN) version of the Risk Management Standard (i.e., EN ISO 14971:2012; http://bit.ly/ISO14971-2012changes), states that “inherent safety by design” is not precise enough. Section 2 of the Essential Requirements (i.e., Annex I of the MDD) states that the first risk control option must be selection of design and construction that eliminates or reduces risk as far as possible, while the international (ISO) risk management standard (i.e., ISO 14971:2007) only states that inherent safety by design is required.
The difference between the requirements of the ISO and the EN standard are not just semantics. If you read part II of the Essential Requirements (ERs; i.e., ER 7-13), there are many examples of how the construction of devices should be considered. The following are three examples:
- ER 7.5 – leaking from the device
- ER 8.2 – tissues of animal origin
- ER 9.2 -aging of materials
Therefore, in order to comply the the intent of the Directive, you must consider far more than just the design of the device. Construction is interpreted as both the risks associated with the materials to fabricate a device and the methods of manufacture. In the proposed EU regulations, the European Commission seeks to clarify the requirements for implementation of risk controls, but the draft legislation still seems vague.
Implementing Risk Control Options for Medical Devices
The following wording for implementation of risk control options in the new proposed second Essential Requirement is below:
“The manufacturer shall apply the following principles in the priority order listed:
a. identify known or foreseeable hazards and estimate the associated risks arising from the intended use and foreseeable misuse;
b. eliminate risks as far as possible through inherently safe design and manufacture
c. reduce as far as possible the remaining risks by taking adequate protection measures, including alarms; and
d. provide training to users and/or inform users of any residual risks.”
In this proposed wording, the word “construction” was replaced by the word “manufacture.” However, in other parts of the new proposed Essential Requirements (http://bit.ly/NewERCGap) the materials of fabrication are specifically addressed, as well. For example:
- ER 7.1d) was added as a new requirement…”d) the choice of materials used, reflecting, where appropriate, matters such as hardness, wear and fatigue strength.”
- ER 7.6 was added as a new requirement to address risks associated with the size and properties of particles—especially nanomaterials.
The new proposed Essential Requirements also include numerous examples of how the manufacturing processes must ensure proper safety. Essential Requirement 10 specifically references new Commission Regulation (EU) No 722/2012 (http://bit.ly/AnimalTissueReg)–specific to devices manufactured using animal tissues or cells of animal origin.
Even though the proposed regulations are more detailed with regard to application of risk management, they do not specify if it is required to implement risk control options for both materials and methods of manufacture simultaneously, or if the manufacturer may choose between the two. The phrase “taking account of the generally acknowledged state of the art” is used in the second Essential Requirement, but “state of the art” is a moving target, and the European Commission may find existing Standards to be deficient.
For reducing the risk of infection, the Commission does not require that companies implement aseptic processing, antimicrobial materials and terminal sterilization. One of the three is sufficient. This is why we have ISO Standards for sterilization validation, and we define “sterile” as a sterility assurance level of 10-6.
If the Commission maintained the language of the ISO 14971:2007 Standard, “as low as reasonably practicable,” then manufacturers could select risk control options based upon acceptability of risk. However, the EN version of the risk management standard creates significant challenges for implementation, and we are forced to evaluate the risk control measures we implement against those used by other manufacturers during the process of risk option analysis.
If you are interested in ISO 14971 training, we are conducting a risk management training webinar on October 19, 2018.
Risk Control Options for Medical Devices: Deviation #6 Read More »