This indications for use case study illustrates 510k requirements using the example of a spinal pedicle screw.

Indications for Use Case Study
A hypothetical new client asked me if I could help them with a 510k submission for a new pedicle screw design. The company’s device utilizes a lower-profile version of a traditional pedicle screw that is pre-packaged with rods, hooks, clamps, and nuts. The pre-packaged system is gamma-irradiated, and the product is specially designed for pediatric patients. Unfortunately, the company was only able to find similar designs of pedicle screws that were for adult patients.
Identifying the FDA Regulation – Indications for Use Case Study
The applicable FDA regulation for a pedicle screw product is 21 CFR 888.3070 – pedicle screw spinal system. However, the only indication stated in this regulation is specific to “skeletally mature patients.” Therefore, the indications portion of the regulation does not apply to the subject device for my hypothetical client.
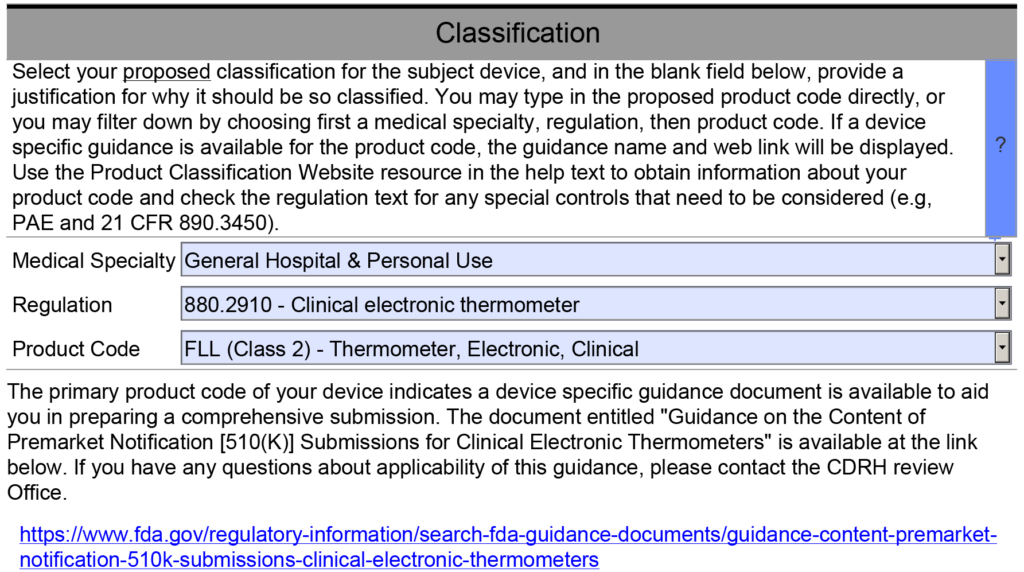
Case Study Product Classification
If you type in “pediatric” as a keyword into the product classification database, 12 different product classification codes result from that search. The applicable product classification code for this product is “OSH”—“Pedicle Screw Spinal System, Adolescent Idiopathic Scoliosis.” The definition for this product classification is: “Intended to stabilize the thoracolumbar spine as an adjunct to fusion using allograft and/or autograft to treat adolescent idiopathic scoliosis.” This is the desired indication because it is specific to pediatric patients. This product classification code also references “pedicle screw spinal system” as the regulation description, and there is a link provided for the 888.3070 regulation number.
Identifying a Predicate Device
97 establishment registration and listing entries are under the “OSH” product classification code. Medicrea Technologies, located in France, submitted one of the most recent 510k submissions (K150049) referencing this product code. Many other possible predicate devices could be used for this 510k submission. However, a recent 510k submission with the same indications for use is usually a good choice. The description of the potential predicate device in the 510k Summary indicates that the predicate is also pre-sterilized. Still, the predicate device description does not specify that it is a low-profile version. Additional research on the company’s website revealed that the PASS LP Spinal System is a low-profile polyaxial spine system. For a 510k submission, it is more important to select a predicate with the same indications rather than a predicate with all the same technological characteristics. However, This particular predicate device appears to have most of the same technological characteristics. The minor differences between the “Pass LP Spinal System” and the subject device are insignificant, and the K150049 was selected as a predicate device submission.
FDA Form 3881
The form has four sections to complete:
- The 510k number is assigned by the FDA to each premarket notification submission (usually not assigned yet).
- The device name is the subject of your 510k submission.
- The indications for use should match the indications for the use of the predicate device, and it should be similar to the indications for use as written in the regulations for the product classification.
- The type of device—prescription-only and/or over-the-counter use.
The form is completed using Adobe Acrobat Pro. The Form used to be a stand-alone document that would be included in Section 4 of the FDA eCopy, but now FDA Form 3881 is incorporated into the FDA eSTAR and PreSTAR templates. The FDA includes this page as part of the 510k Summary published on the FDA website for all 510k-cleared devices. For this case study, the 510k number is unknown. The name of the subject device is the “Miniflex Pedicle Screw Spinal System.” The indications for use are: “The Miniflex Pedicle Screw Spinal System is used for posterior non-cervical pedicle screw fixation in pediatric patients. The spinal implants are indicated as an adjunct to fusion to treat adolescent idiopathic scoliosis. The spinal implants are intended to be used with allograft and/or autograft. Pediatric pedicle screw fixation is limited to a posterior approach.” The device type is for “Prescription Use” only.
Writing Your Indications for Use
When writing an Indications for Use statement, the most straightforward approach is substituting your subject device’s name for the predicate device’s name. Ideally, you have chosen a predicate device that matches your subject device for the indications for use and the technical characteristics. However, it is possible to have a subject device with a narrower indication. For example, the predicate device may be indicated for both adult and pediatric patients, while your subject device may be specifically designed to fit pediatric patients better. For our case study, only the last paragraph of the predicate’s IFU applied to the subject device because the device was limited to pediatric use. Therefore, the last paragraph was copied, and the subject device name was substituted for the predicate device name.
Indications for Use Case Study – Broader Indications
The first step of the 510k review process is verification that the subject device has the same indications for use as the primary predicate device. Therefore, if broader indications for use are claimed for the subject device, then the 510k submission will likely be rejected as not substantially equivalent (NSE). In this case, you have a few options. One alternative for submissions that require a broader indication for use is to perform a clinical study to provide safety and efficacy for the broader indication. A second alternative is to submit a De Novo application.
You should request a pre-submission meeting with the FDA before pursuing either option. In the case of a clinical study, you should plan to provide the FDA with a clinical study synopsis that includes a benefit/risk analysis. The clinical study synopsis should also include a rationale for why the broader indication for use presents a non-significant risk if you plan to conduct the clinical study under good clinical practices (GCPs) instead of applying for an investigational device exemption (IDE). Suppose you plan to submit a De Novo application. In that case, it is recommended that you prepare a special controls guidance document before the pre-submission meeting to obtain feedback from the FDA. For novel devices of medium risk, your company may need to conduct a clinical study and submit a De Novo application.
Your Next 510k Submission
Most companies have plans for subsequent submissions to expand the functionality of the subject device. In this case, often, the subject device is the best choice of a predicate device for subsequent 510k submissions. In this case, you should attempt to make the initial application as broad as possible concerning indications for use. This will enable you to narrow the indications for use in future submissions without the device being NSE to your predicate device.
If you are interested in watching a webinar on the topic of indications for use, please visit the webinar page.
Additional 510k Training
The 510k book, “How to Prepare Your 510k in 100 Days,” is available as an eBook only with the purchase of our on-line 510k course series. Please visit the webinar page to purchase individual webinars.