EN standard – What is it?
CE Marking auditors may ask if you assessed the difference between the ISO version of a standard and the EN standard. Is there a difference?
What is an EN standard?
European Standards are technical standards ratified by one of the three European standards organizations: CEN, CENELEC, or ETSI. European Standards are referred to as an “EN standard.” The “EN” is derived from the German name Europäische Norm (i.e., translated as “European Norm”). Each of the member states have their own standards organizations that is responsible for adopting ISO standards that have been ratified, translation of the standard into the language of the member state, and and issue of translated EN standard. For example: German standards are preceded by “DIN,” Irish standards are preceded by “I.S. EN,” and Swedish standards are preceded by “SS-EN.”
How is an EN version different from the ISO version of a standard?
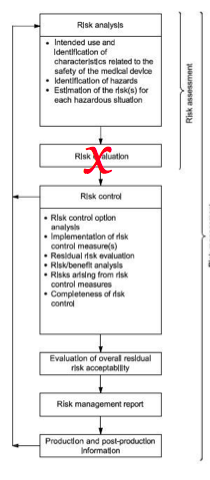
Historically, EN medical device standards include three Annexes related to harmonization with the standard with the three EU Directives (i.e., MDD, AIMD, and IVDD). Now that the EU Device Regulations have been released (i.e., MDR = 2017/745) and (IVDR = 2017/746), the EN standards now have harmonization Annexes for the two regulations (i.e., ZA and ZB). The content of the ISO Standard is usually not changed in an EN standard unless there is a correction, amendment, or deviation.
Where can you purchase EN versions?
EN standards are translated from the adopted ISO standard by the standards organization for each member state. Below are a few examples:
- Swedish – Swedish Institute for Standards
- Irish – National Standards Association of Ireland
- German – German Institute for Standardization
- Estonian – Estonian Center for Standards and Accreditation
If you already own the ISO version, do you need to buy the EN standard too?
In a Technical File for CE Marking, you are required to demonstrate how you comply with Annex I of the MDR or IVDR. The recommended method of demonstrating compliance is creating a General Safety and Performance Requirements (GSPRs) checklist. In that checklist, you need to identify which applicable standards were used. If an EN standard is available, the GSPR checklist should reference that standard instead of the ISO version. Unfortunately, in order to claim compliance with the EN version, someone needs access to that standard. This could be within your organization or a consultant that assisted in preparing the GSPR checklist to ensure that you are compliant with the EN standard. Generally, we purchase English versions of EN standards from the Estonian Center for Standards and Accreditation, because it is usually the least expensive source. However, if you ask a consultant to do this comparison for you, the best way to perform that comparison is using the comparison function of Adobe Acrobat Pro.
Can you identify EN requirements without the EN version?
Most of the quality system requirements for the MDR and IVDR regulations are found in Article 10 of the regulation. However, there are quality system requirements found in other articles and in the annexes. Therefore, you may be able to find EU requirements in the regulations by doing a keyword search. For example, searching for the word “risk” may help you find risk management requirements throughout the regulations. You may also find European-specific requirements in Common Specifications and MDCG guidance documents.
How to respond to a certification body auditor?
Final Answer: I’m not sure, because every auditor is a little different in their approach. However, as an instructor, I would teach an auditor to ask open-ended questions, such as: “How did you determine if there is an impact upon your procedures and design documentation with regard to the EN standard?” (i.e., – impact analysis). If the company provides an impact analysis and explains why the existing documentation and procedure should not change, I believe this meets the EU requirements. If the certification body auditor is still not satisfied, then you might try asking them, “What differences are you aware of between the EN and ISO versions of the standard?”
EN standard – What is it? Read More »