4 Ways to Make the Best Use of Medical Device Remote Audits
This blog identifies how to use medical device remote audits effectively, save time and resources, and when you should not conduct audits remotely. Most audits ISO 13485 are performed onsite at the location where the processes are being performed, and are the most effective approach to internal and supplier audits. But conducting an audit from your desk makes more efficient use of your time as an auditor. A large percentage of audits are conducted from conference rooms where the auditor spends an excessive amount of time reviewing documents and records, or waiting for documents and records to be delivered.
Most audits ISO 13485 are performed onsite at the location where the processes are being performed, and are the most effective approach to internal and supplier audits. But conducting an audit from your desk makes more efficient use of your time as an auditor. A large percentage of audits are conducted from conference rooms where the auditor spends an excessive amount of time reviewing documents and records, or waiting for documents and records to be delivered.
In 2006, the first edition of the ISO 17021 standard for certification of quality systems by certification bodies was released. ISO 17021 requires that initial certification audits be conducted in two stages. Stage 1 has several requirements, but the first element of Stage 1 is reviewing quality system documentation. In most cases, Stage 1 and Stage 2 audits are conducted onsite. Still, if the auditee is located in a remote location (such as New Zealand), Stage 1 audits will sometimes be conducted via conference call.
Prior to ISO 17021, a review of quality system documentation was the only task performed before the initial certification audit, and the documentation review was typically conducted remotely as a “desktop” audit. Desktop audits have been used for decades as a way of auditing quality system documentation without traveling. However, desktop audits can be much more than a review of quality system documentation. You can interview auditees on the phone, review records, even ask auditees to demonstrate activities in real-time using a web camera.
Documentation can also consist of much more than text. Raw data, statistical analysis, and photos can be used to communicate additional information. The more multimedia content provided to auditors remotely, the closer a remote audit becomes to auditing on site. The same requirements as certification bodies do not bound internal auditors and supplier auditors, and audits may be conducted onsite or remotely. The most recent version of ISO 19011 (2011), includes a comparison table for onsite and remote auditing in Annex B.
Medical Device Remote Supplier Audits
The use of remote audits to qualify suppliers is not recommended for four reasons:
- onsite visits facilitate the building of supplier-customer relationships
- touring facilities and watching a demonstration of processes improves understanding of a supplier’s processes better than reading documents and records can
- Cleanliness and capabilities of suppliers are best evaluated onsite, where camera angles can be used to crop out important details
- sometimes suppliers misrepresent their capabilities by showing photographs on their website of other companies.
After you have qualified a supplier, however, you may not need to audit them onsite regularly. If a supplier’s performance is good and risks associated with nonconforming components supplied are minimal, then you have a justification for conducting a remote audit. However, if a supplier’s performance is poor, you may want to use a remote supplier audit as a precursor to an onsite supplier audit to investigate the reasons for nonconforming components (i.e., a “for cause” audit). Regardless of the situation, the amount of time spent in your supplier’s conference room should always be by reviewing documents and records remotely. This will reduce the amount of time required at each supplier, and enables you to audit two suppliers during the same trip.
Medical Device Remote Internal Audits
It might not occur to you that there would be any need for remote internal audits. However, not all internal audits are performed by a person working at your location. Larger companies have multiple sites, and many of the internal audits are performed by auditors from corporate headquarters and other locations. In the case of internal audits performed by auditors from other locations, travel time can be minimized by performing part or all of the internal audits remotely. This approach can also work for consultants hired to conduct internal audits. There is no need to spend money on the cost of travel for a consultant if the consultant is only going to be auditing documents and records. The following are great examples of processes that can be audited remotely:
- CAPA
- Management Review
- Internal Auditing
- Supplier Controls
- Complaint Handling
- Adverse Event Reporting
Medical Device Remote Re-audits
21 CFR 820.22 indicates that re-audits may be required where corrective actions have been taken to verify the effectiveness of the actions taken: “Corrective action(s), including a re-audit of deficient matters, shall be taken when necessary.” However, if nonconformities identified during an audit are categorized as “high-risk,” it may be essential to conduct a verification of corrective action effectiveness as soon as possible.
Sometimes, effectiveness can be determined by reviewing quantitative metrics. Still, if a re-audit is needed, then a remote re-audit may allow the auditor to verify the effectiveness of corrective actions without the necessity of being onsite. If verification of corrective action effectiveness can be performed by reviewing documents and records, a remote re-audit is appropriate. Other corrective actions, especially those involving production and process controls, typically require onsite verification.
Remote Audit Team Members
Most medical device companies have a limited number of qualified auditors, and auditing is almost always a secondary job duty. However, audits often require specific technical knowledge that only one or two auditors may possess. Therefore, it may be extremely difficult to schedule a team audit when all the required auditors and auditees are available. There is another option to postponing your audit. You might consider having some of your auditing team members audit remotely from their desks, while the rest of the team conducts an onsite audit. For example, most lead auditors can conduct a process audit of incoming inspection, storage, and shipping. However, auditing surface mount assembly lines for the fabrication of printed circuit boards requires more technical knowledge of this type of process. Technical expertise is also needed to audit sterilization or CNC machining.
By working together, onsite audit team members can take directions from a technical subject matter expert working remotely and gather information needed to audit any process properly. This approach minimizes time requirements for subject matter experts, and remote audits by team members reduce the cost of travel.
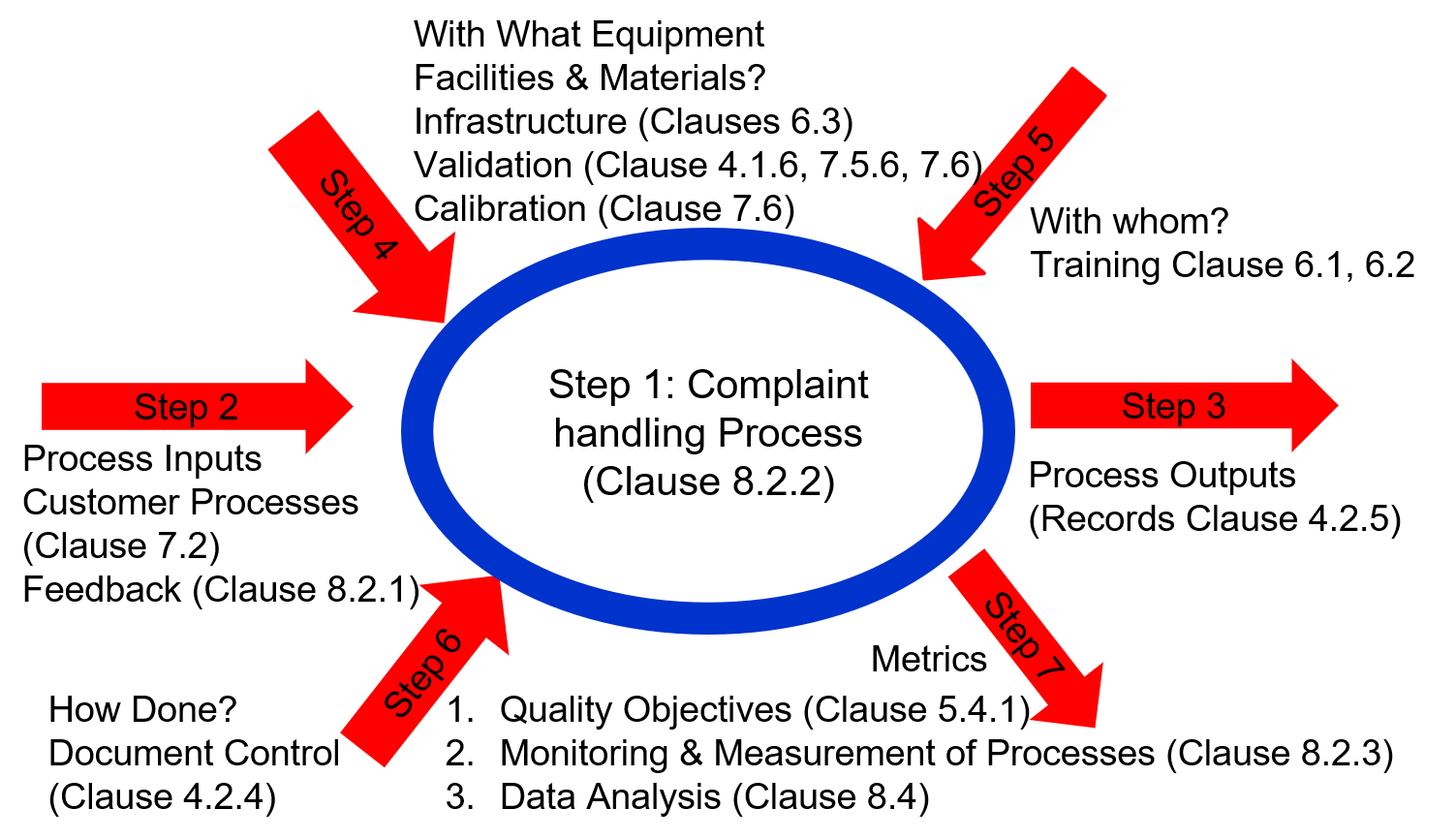
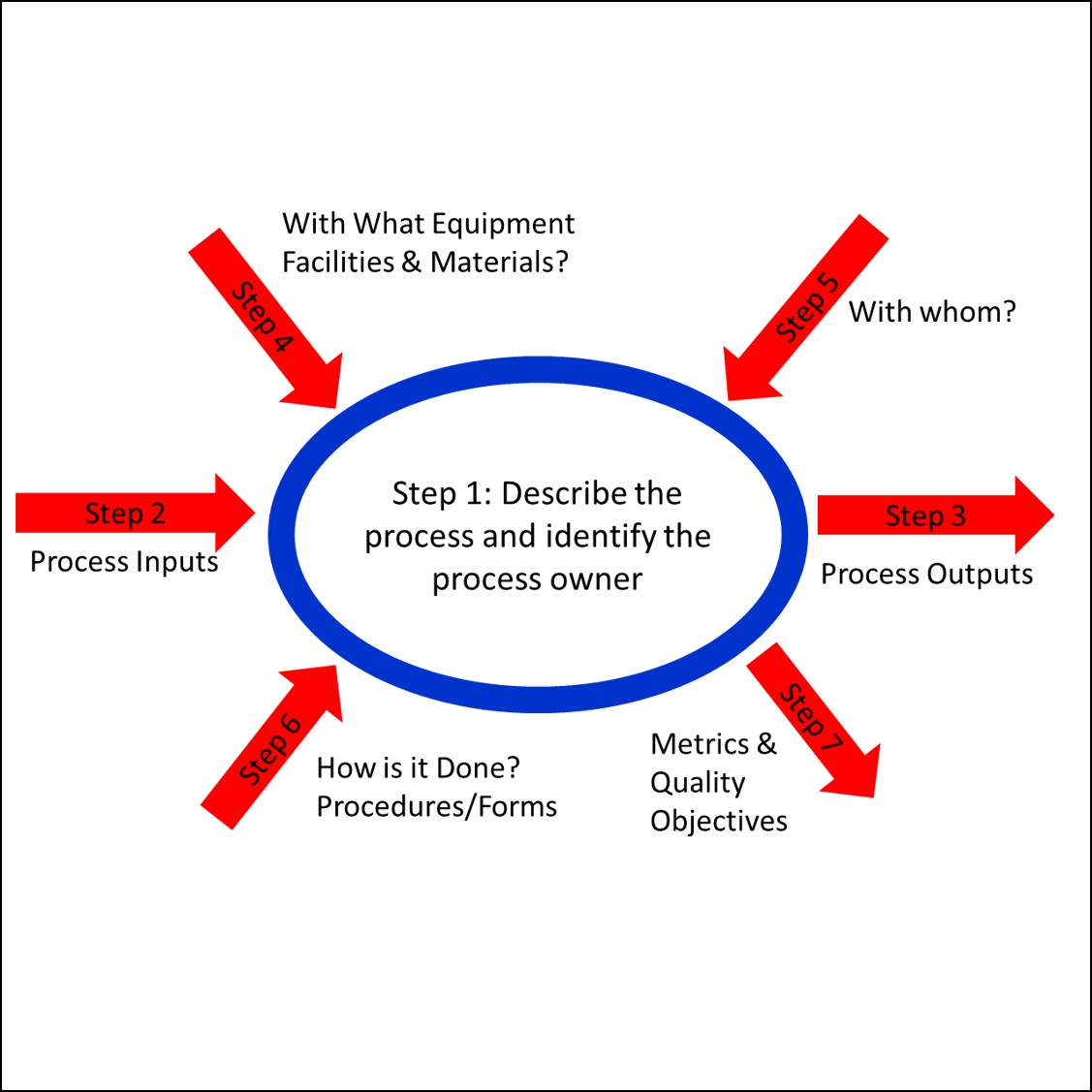
If you are interested in learning more about Turtle Diagrams and the process approach to auditing, please register for our webinar on the process approach to auditing. If you are interested in learning more about how you can use remote audits to save time and money, please contact us. We can help you identify immediate opportunities.
4 Ways to Make the Best Use of Medical Device Remote Audits Read More »