Cybersecurity FDA Guidance for Devices with Software and Firmware
This article reviews the 2014 FDA guidance for premarket and post-market cybersecurity of medical devices with software and firmware—including requirements for reporting field corrections and removals.

Hospitals, home health systems, and medical devices are more connected now than ever. The automatic communication between medical devices and network systems is improving efficiency and accuracy in the world of healthcare. Medical devices are capable of more computing, analysis, reporting, and automation to improve the speed and quality of patient care. There are even devices that consist only of software (i.e. software as a medical device or SaMD). Along with technological advances, new risks and concerns are also introduced. The risk of hackers exploiting vulnerabilities in networks and software is inevitable. The FDA introduced guidance for both pre-market and post-market cybersecurity to assist manufacturers in developing effective controls to protect patients and users. Cybersecurity protection requires Identification, Protection, Detection, Response, and Recovery.
The first step is incorporating processes and procedures to improve device cybersecurity into your quality management system. You should have a specific cybersecurity plan (i.e. security risk management plan) to outline the steps necessary to ensure a safe and secure medical device. In addition, your software development team will need cybersecurity training. The only medical device guidance document specific to cybersecurity is currently AAMI TIR57:2016.
Identify Cybersecurity Risks
The key to understanding and assessing the cybersecurity risks involved with your device begin in the early stages of design development. At the start of the risk management process, you need to identify the essential safety and performance requirements of the device. You need to identify any potential cybersecurity vulnerabilities that could impact safety or performance, as well as the specific harms that could result if the vulnerability was exploited. In assessing the specific vulnerabilities, the FDA recommends using the Common Vulnerability Scoring System (CVSS). There is a CVSS calculator available online through NIST. The overall score is calculated based on different factors such as attack vector (local, adjacent network, network), access complexity (high, medium, low), authentication (multiple, single, none), the impact of confidentiality (none, partial, complete), exploitability (unproven that exploit exists, proof of concept code, functional exploit exists), remediation level (official fix, temporary fix, workaround, unavailable), collateral damage potential (low, medium, high), etc. This score is used in the hazard analysis in determining the level of risk.
Cybersecurity Protection
The process of assessing the exploitability and harms can also assist in determining mitigations that can be implemented to reduce the cybersecurity risk. During the design process, the FDA expects you to implement as many protections as practicable. Protections include:
- Limit Access to Trusted Users
- Password protection strengthened password requirements
- User authentication
- Layered privileges based on user role
- Limit Access to Tampering
- Physical locks on devices and/or communication ports
- Automatic timed methods to terminate sessions
- Ensure Trusted Content
- Restrict software or firmware updates to authenticated code
- Systematic procedures for authorized users to download software and firmware only from the manufacturer
- Ensure capability of secure data transfer, use of encryption
Cybersecurity Detection
The FDA also requires you to implement features that allow for security compromises to be detected, recognized, logged, timed, and acted upon during regular use. You should develop and provide information to the end-user concerning appropriate actions to take upon the detection of a cybersecurity event. Methods for retention and recovery should be provided to allow recovery of device configuration by an authenticated privileged user.
If you include off-the-shelf (OTS) software in your device, you are responsible for the performance of the software as part of the device. All software changes to address cybersecurity vulnerabilities of the OTS software need to be validated. You need to maintain a formal business relationship with the OTS vendor to ensure timely notification of any information concerning quality problems or corrective actions. Sometimes you will need to involve the OTS vendor to correct cybersecurity vulnerabilities.
Post-Market Surveillance
Once you complete the hazard analysis, mitigation implementation, validations, and has deployed their device for use – your activities shift to post-market management. Several QMS tools can assist in the cybersecurity processes post-market, including complaint handling, quality audits, corrective and preventive action, ongoing risk analysis, and servicing. A critical component of every cybersecurity program is the monitoring of cybersecurity information sources to assist in the identification and detection of risk. You should maintain contact with third-party software suppliers for the identification of new vulnerabilities, updates, and patches that come available.
There are many sources that companies should follow for information relating to cybersecurity, including independent security researchers, in-house testing, software or hardware suppliers, healthcare facilities, and Information Sharing and Analysis Organizations (ISAO). Involvement in ISAOs is strongly recommended by the FDA and reduces your reporting burden if an upgrade or patch is required post-market. ISAOs share vulnerabilities and threats that impact medical devices with their members. They share and disseminate cybersecurity information and intelligence pertaining to vulnerabilities and threats spanning many technology sectors, and are seen as an integral part of your post-market cybersecurity surveillance program.
Response and Recovery
If you identify a cybersecurity vulnerability, there are remediation and reporting steps that need to occur. Remediation may involve a software update, bug fixes, patches, “defense-in-depth” strategies to remove malware, or covering an access port to reduce the vulnerability. Uncontrolled risks should be remediated as soon as possible and must be reported to the FDA according to 21 CFR 806. Certain circumstances remove the reporting requirement. The decision flowchart below can be used to determine the reporting requirements.
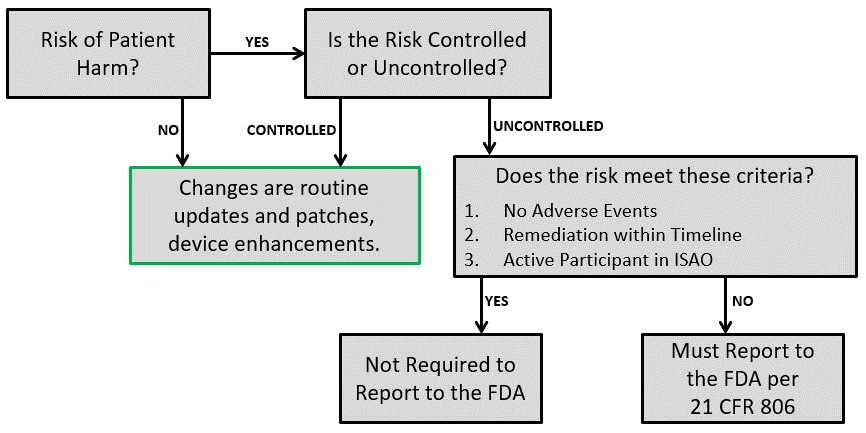
In addition to reporting corrections and removals, the FDA identifies specific content to be included in PMA periodic reports regarding vulnerabilities and risks. If you have a Class III device, you should review that section thoroughly to ensure annual report compliance.
If a device contains software or firmware, cybersecurity will be an important component of the risk management processes, and continual cybersecurity management will be necessary to ensure the ongoing safety and effectiveness of your device. If you need more help with cybersecurity risk management of your medical device, please schedule a free 30-minute call with Medical Device Academy by clicking on the link below.
Cybersecurity FDA Guidance for Devices with Software and Firmware Read More »