Contract Manufacturers Need Strong Risk Management Processes
This blog discusses why contract manufacturers need to have a strong risk management process, and your company needs to help your contract manufacturers. This article was updated on April 28, 2022, and the original publication was January 05, 2011. Please ignore the date of publication at the top of the post for articles that are more than a year old.

Can contract manufacturers exclude risk management from the scope of their quality system?
Most contract manufacturers in the medical device industry exclude design from their Quality Management Systems. Unfortunately, most of the contract manufacturers also associate risk management with only the design process. Risk Management cannot be “not applicable” in an ISO 13485 Quality Management System. The requirement of section 7.1 is: “The organization shall establish documented requirements for risk management throughout product realization. Records arising from risk management shall be maintained.” The Standard also references ISO 14971 as a source of guidance on Risk Management.
Medical Device Academy also offers a Two-Part Risk Management Training Webinar for ISO 14971:2019.
Have you experienced an audit dialogue at a contract manufacturer similar to this?
The auditor asks, “How do you manage risk throughout the production process?” Then the auditee responds, “That is the responsibility of our customers. We will prepare a risk analysis if customers pay for it, but usually, customers do the risk analysis.”
For a contract manufacturer, compliance with ISO 14971 is not my primary concern as an auditor. My primary concern is to verify that contract manufacturers analyze risks associated with the processes that they perform and do their best to minimize those risks. What I don’t understand is why more companies don’t want to have strong risk management processes. Risk management is how we prevent bad things from happening. Bad stuff like scrap, complaints, and recalls. Should we expect our suppliers to have a strong risk management process?
Duh.
Why your company needs to be involved in the risk management process?
Contract manufacturers should be doing everything they can to get better at risk management. During pre-production planning, they should be asking, “What happens if…” The contract manufacturer knows best HOW things will fail in production, while the customer knows best WHAT happens when things fail in production. To be safe and effective, both companies need to collaborate on risk analysis.
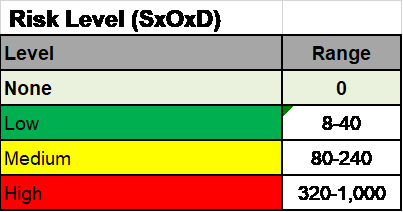
In any risk analysis, you need to estimate the severity of potential harm and the probability of occurrence of that harm. For production defects, the contract manufacturer can estimate the probability of occurrence of defects (i.e., P1 in Annex E of ISO 14971:2007), but the likelihood of occurrence of harm is less. The probability of occurrence of harm is the product of multiplying P1 and P2. The probability that occurrence will result in harm is P2, and P2 is a number that is less than 100% or 1. Your company can gather pre-market clinical data and post-market clinical data to estimate P2, but before launching your product, you can only guess at the value of P2. Your contract manufacturer, however, is not able to estimate P2 at all. It’s ok to estimate risk without P2 during the design phase because this will overestimate risks and result in more conservative decisions.
In addition to P2, your contract manufacturer is also not capable of estimating the severity of potential harm. As the designer of the medical device, you will know best how your device is used and what the likely clinical outcomes are when a device malfunctions. There may even be multiple possible clinical outcomes. The contract manufacturer knows what can go wrong during manufacturing, but you will need to define the clinical outcomes due to malfunctions.
Why do contract manufacturers avoid doing risk analysis?
The reason contract manufacturers avoid doing risk analysis is because it’s time-consuming and tedious.
Too bad, so sad.
Balancing my checkbook is time-consuming and tedious too, but I balance my checkbook to prevent an overdraft charge. Not doing a risk analysis can be much more painful. Scrapping out a part can cost tens or hundreds of dollars. Complaints can cost thousands of dollars. Recalls can cost millions of dollars.
If I owned a contract manufacturing company, I would ensure that everyone in the company is involved in risk management. We don’t want scrap, we can’t afford mistakes that lead to complaints, and a recall could put us out of business.
How Medical Device Academy Can Help?
Medical device academy can help both the contract manufacturer and the specification developer utilizing a contract manufacturer as a supplier! We offer training on 14971:2019 as well as procedures on risk management and supplier quality management.
Two-part Risk Management Training Webinar for ISO 14971:2019 – Part 1 of this webinar will be presented live on Tuesday, March 29 @ 9-10:30 am EDT. Part 2 of this webinar series will be presented live on Tuesday, April 5 at 9-10:30 am EDT. Purchase of this webinar series will grant the customer access to both live webinars. They will also receive the native slide decks and recording for the two webinars.
Contract Manufacturers Need Strong Risk Management Processes Read More »