Risk Control Selection – Deviation #5 in ISO 14971
ISO 14971:2012 deviation #5 is specific to selecting risk control options and protective measures for CE Marking medical devices.
 If your company is CE Marking medical devices, you are required to satisfy the Essential Requirements for Safety and Performance as defined in the three European Directives: the MDD, the AIMD, and the IVDD. Throughout these Essential Requirements, there is a requirement to reduce risks “as far as possible” (AFAP) by implementing risk controls. At one time, the expectation was for companies to implement state of the art concerning risk controls, and “state of the art” was interpreted as the latest version of the harmonized ISO Standards. However, lawyers dominating the European Commission appear to disagree with the status quo.
If your company is CE Marking medical devices, you are required to satisfy the Essential Requirements for Safety and Performance as defined in the three European Directives: the MDD, the AIMD, and the IVDD. Throughout these Essential Requirements, there is a requirement to reduce risks “as far as possible” (AFAP) by implementing risk controls. At one time, the expectation was for companies to implement state of the art concerning risk controls, and “state of the art” was interpreted as the latest version of the harmonized ISO Standards. However, lawyers dominating the European Commission appear to disagree with the status quo.
Therefore, in 2012, the European National (EN) version of the Medical Device Risk Management Standard was revised. There is no change to the content of Clauses 1 through 9. Instead, the European Commission identified seven content deviations between the ISO 14971 Standard and the EU Directives. These deviations are identified and explained in Annexes ZA, ZB, and ZC. This blog is the fifth installment of Medical Device Academy’s seven-part blog series on this topic. The goal of the series is to identify solutions for meeting the Essential Requirements by suggesting changes to the current best practices of implementing a risk management process for medical device design.
Discretion as to the Risk Control Options/Measures
Essential Requirements 1 and 2 require that risk control options are implemented for all risks before determining the acceptability of residual risks. The 2nd Essential requirement also requires manufacturers to implement all risk control options—unless the risk controls do not further reduce risk.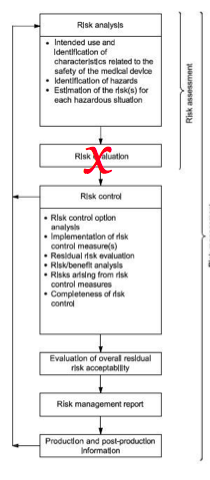
Clause 6.2 of the 14971 Standard suggests that you only need to use “one or more” of the risk control options, and Clause 6.4 indicates that further risk control measures are not required if the risk is acceptable. There is an apparent contradiction between the intent of the Standard and the Directives.
If risk acceptability has no impact on whether you will implement risk controls, there is no need for performing a preliminary risk evaluation. Therefore, I have three recommendations for changes to your current risk management process:
- Ignore Clause 5 of the 2007/2009 version of ISO 14971
- Eliminate the second step of risk assessment from your flow chart for risk management (see Figure 1 from the 14971 Standard)
- Define risk management policies upon clinical benefits, rather than absolute risks
Instead of performing a preliminary risk evaluation (Clause 6.5), risk/benefit analysis should be moved to Clause 7, where the evaluation of overall residual risk acceptability is required. By making this change, risk controls will be implemented, regardless of risk acceptability, and the acceptability of risks will be dependent upon the risk/benefit analysis alone.
Impact of this Deviation
Implementing changes to your risk management process to address this deviation has great potential to impact the design of devices—not just the risk management documentation. Design teams will no longer be able to stop the design process with an initial design that has an “acceptable risk.” Instead, design teams will be forced to implement additional risk controls and protective measures for device designs that already have a low risk of harm for specific failure modes.
The requirement to implement additional risk controls will increase the cost of devices that may have been relatively safe without the risk controls. For example, if a device is not intended to be implanted, it is a potential foreseeable misuse. Your company may have used the instructions for use to communicate the residual risk associated with misuse of the device. However, now your company will have to implement design controls (e.g., –a selection of materials suitable for implantation) to eliminate the risks associated with misuse and protective measures (e.g., – radio-opaque thread) to help retrieve product that was implanted in an “off-label” usage.
If you are interested in risk management training, Medical Device Academy offers a risk management training webinar.
Risk Control Selection – Deviation #5 in ISO 14971 Read More »


