The FDA CCP keeps getting better. Now, you can request small business determination (SBD) online through the FDA CDRH portal.
Preparing your FDA eSubmission for the FDA CCP
There are three possible formats for an FDA eSubmission to the FDA CCP for CDRH: 1) FDA eCopy, 2) FDA eSTAR, and 3) Small Business Determination (SBD) Request. We explain each in sequence below:
FDA eCopy:
The FDA eCopy is no longer an acceptable format for a 510k submission. You must use the FDA eSTAR template for a 510k submission. In contrast, pre-submission meeting minutes and withdrawal letters require an FDA eCopy. An FDA eCopy is also required for Sprints (i.e., pre-sub variation for Breakthrough devices and STeP devices). For a De Novo Submission and 513(g) submissions, you have the option of using the FDA eSTAR or the FDA eCopy (BUT use the FDA eSTAR or PreSTAR respectively). Here are the preparation steps:
- Confirm your eCopy complies with FDA’s eCopy guidance.
- Compress your eCopy into a “.zip” file.
FDA eSTAR (also includes FDA PreSTAR):
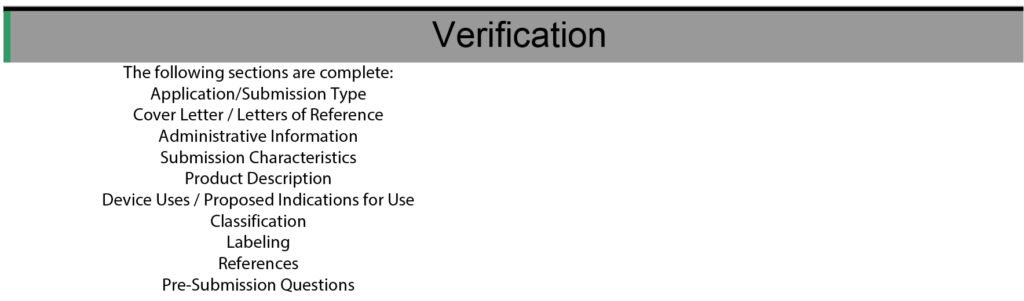
Confirm your eSTAR is complete. There are three ways to know that your eSTAR is complete and ready for upload, but don’t worry too much. The FDA validated that the FDA CCP will automatically detect an incomplete eSTAR and will not allow it to be uploaded.
- Bold Font at the top of the eSTAR or PreSTAR template is Green

- All of the color-coded bars on the left side of the template are Green or Grey

- All of the sections of the template in the verification section are found on the left side
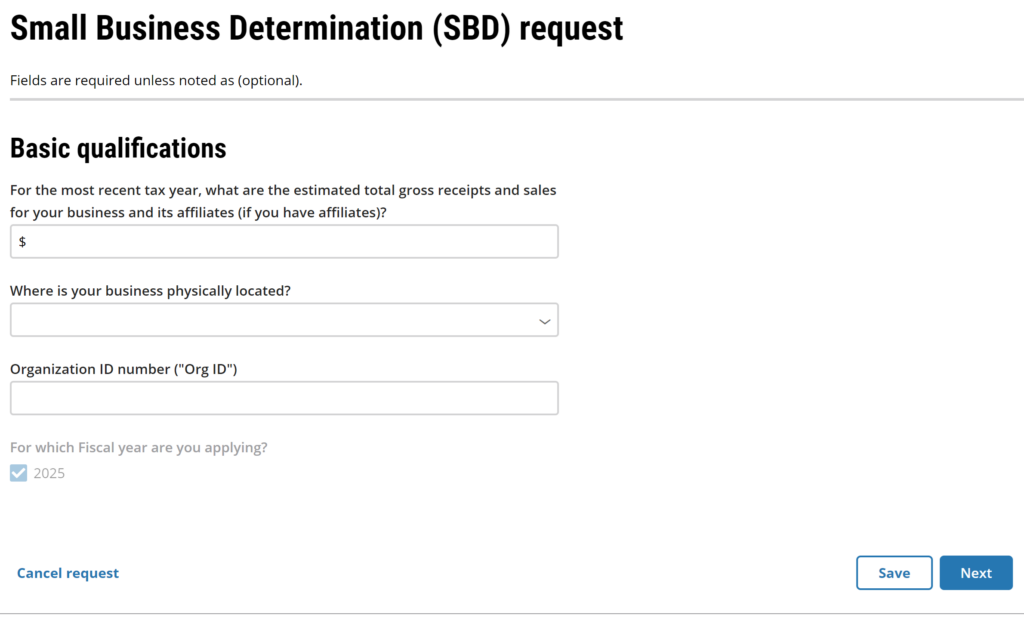
Small Business Determination Request:
The ability to upload your small business qualification documents as an eSubmission instead of sending a hardcopy via courier is a new feature enabled by the FDA on Saturday night (i.e., September 29, 2024). Confirm your eCopy complies with FDA’s eCopy guidance.
FDA CCP step-by-step uploading process
When you are uploading a submission to the FDA CCP (i.e., CDRH Customer Collaboration Portal), you must perform the following steps:
#1 Sign in to the portal on the login page. If you don’t already have your own account, you can Sign up in less than 5 minutes.
#2 Accept the FDA User Terms and Conditions

#3 You have three options one you accept the FDA user terms and conditions. One option is to check on the status of prior eSubmissions on the Home page. Your second option is to send a new FDA eCopy or FDA eSTAR. For this second option you click on the “+” symbol on the left panel of the webpage (if you hover over the “+” symbol, you will see “Send a submission”) or you can expand the left panel as I have done in the picture below. The third option (i.e., the newest FY2025 option) is to create a request for small business determination (i.e., SBD).
#4 If you selected “Send a submission” you can upload your FDA eCopy or FDA eSTAR. The top of the page has the following instructions “Send your submission before 16:00 ET on a business day for us to process it the same day. Once you send a submission through the portal, you do not need to physically ship any copies to FDA. The online process replaced the physical process.”
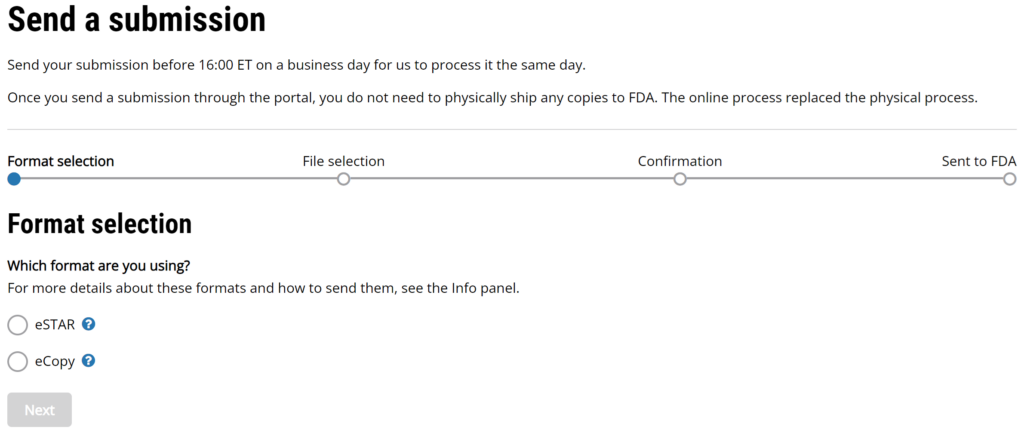
#5 Click on the “Next” button that appears below the selection formats once a format is selected.
#6 Drag & drop your single “.zip” file here, or browse for it.
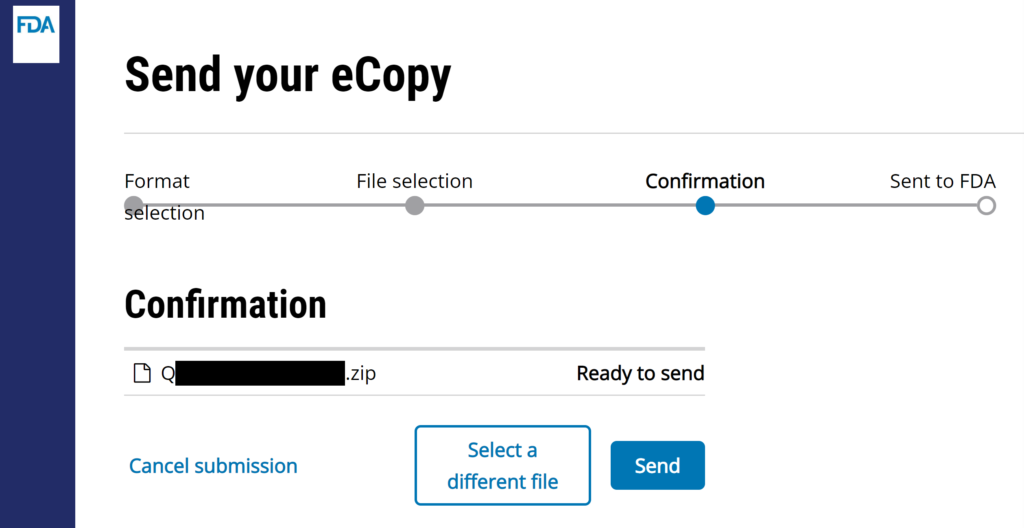
#7 The webpage will ask you for a short description of your submission. The company name and type of submission is sufficient [e.g., “Medical Device Academy – presubmission meeting minutes (i.e., Q24xxxx.A001)”]. Click on “Send” button to complete the uploading process.
#8 Verify that the FDA CCP site gives you a confirmation for the successful uploading of your submission. I always create a screen capture of the confirmation page once I hit send, but that is unnecessary but faster to insert in an email to your boss. You will receive an email seconds later.
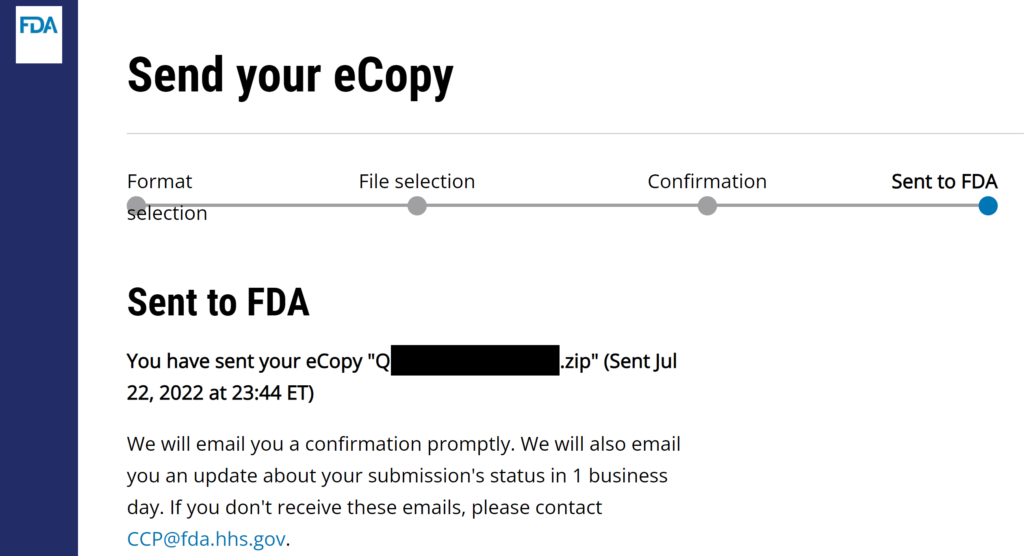
The process is identical for the FDA eSTAR, but you DO NOT need to zip an FDA eSTAR or PreSTAR.
Starting a Small Business Determination (SBD) Request
The third type of submission for the FDA CCP is a small business determination. This new option was enabled last Saturday night (i.e., September 28, 2024)….what a thoughtful birthday present from the FDA to me. Just click on the document icon beneath the plus sign. The website will take you to the “Create a request” page where you can begin your SBD request. Before you start this process, you will need to prepare some documents:
#1 Tax return for the most recent tax year
#2 Organization ID number (watch our YouTube Video)
#3 Other information on FDA Form 3602 (see our Small Business Qualification webpage)

July 2022 Update for the FDA eCopy process (FDA CCP is launched!)
Finally, we can use the new FDA CCP to eliminate FedEx shipments, and 100% of your submissions will be electronic through the portal. The FDA created a Customer Collaboration Portal (CCP) for medical device manufacturers. Initially, the portal’s purpose was to provide a place where submitters could track the status of their submissions and verify the deadlines for each stage of the submission review process. On July 19, 2022, the FDA emailed all active FDA CCP account holders that they can upload both FDA eCopy and FDA eSTAR files to the portal 100% electronically. The FDA released a draft eSTAR guidance as well. Since our consulting team sends out submissions daily, everyone on the team was able to test the new process. If you have a CCP account, you no longer need to ship submissions via FedEx to the Document Control Center (DCC).
FDA Q&A about the new FDA CCP Submission Uploading Process
- Medical Device Academy Question: Who will be permitted to use the FDA CCP to upload submissions for the DCC? FDA Response: We will first offer this feature in batches to people like you who already use CCP so we can study its performance. We will then refine it and make it available to all premarket submitters.
- Medical Device Academy Question: What do you need to use the FDA CCP? FDA Response: You don’t need to do anything to participate since you already use CCP. We will email you again when you can start sending your next submissions online.
- Medical Device Academy Question: Suppose another consultant asks me to submit an eSTAR or eCopy for them, or I do this for a member of my consulting team. Is there any reason I cannot upload the submission using my account even though the other person is the official submission correspondent and their name is listed on the cover letter? FDA Response: The applicant and correspondent information of the submission is still used when logging the submission in. The submitter (i.e., the person uploading the submission) is not used in any part of the log-in process. The submission portal is essentially replacing snail mail only; once the DCC loads the submission, whether it be from a CD or an online source, the subsequent process is identical to what it used to be, for now.
- Medical Device Academy Question: Is there any type of eCopy that would not be appropriate for this electronic submission process (e.g., withdrawal letters, MAF, or breakthrough device designations)? FDA Response: You can use the eCopy option to submit anything that goes to the DCC, so all your examples are fair game, though interactive review responses would still be emailed to the reviewer.
- Medical Device Academy Question: How can I get help from the FDA? FDA Response: If you have questions, contact us at CCP@fda.hhs.gov.

Pingback: What is an FDA Breakthrough Device Designation? Medical Device Academy
Pingback: 510k Electronic Submission Guidance for FDA 510k Submissions Medical Device Academy
Pingback: eCopy Guidance is Finally Updated by FDA - Medical Device Academy Medical Device Academy
Hello Rob,
This is Yuki Gomi. It is very nice to talk to you and your blog is really helpful.
I am working as RA staff in the medical device manufacturer and responsible for the FDA as well.
I have quick questions about 510(k) submission to the FDA.
No.1
will CCP be sufficient when submitting 510(k) in eSTAR to the FDA even after October 1, 2023 ?
There are 2 ways to make 510(k) submission to the FDA.
One is the submission through CCP as your blog mentions.
The other is via Electronic Submissions Gateway (ESG).
I only have the account of CCP, but I think CCP is enough in terms of 510(k) submission.
No.2
What is the difference between CCP and ESG ?
In my rough understanding, CCP is limted and ESG covers more.
Hi Yuki,
Great question. Thank you.
The CCP is required after October 1, 2023. You will no longer be permitted to submit a USB via the mail or courier. Everyone should use the CCP account and eliminate waste of USB and mailing.
The CCP is a portal designed by CDRH for communication with pre-market submitters and to upload documents. The ESG is for MDRs, PMAs, etc. The ESG requires a webtrader account for small submitters. Both CCP and ESG are electronic.
Pingback: FDA User Fees for FY 2024 released on July 28, 2023 - Medical Device Academy Medical Device Academy