This procedure bundle is our updated design control procedure for compliance with 21 CFR 820.30 and ISO 13485:2016, Clause 7.3.1.
Design Controls Procedure
The purpose of this design controls procedure is to ensure that the product is developed in a systematic way, ensuring that risk control measures are incorporated in the design, that all design outputs are verified against specifications and validated against user requirements, and that regulatory and standards requirements are fulfilled. The procedure is also bundled with a webinar that explains how to review, edit, and implement the procedure for your company. In the webinar we will explain what order to use the templates and forms in a design project. We are also making some edits to the procedure, forms, and templates in preparation for the live webinar.
When is the live webinar scheduled for this procedure bundle?
The live webinar is scheduled for Monday, September 16, 2024 @ 10:30 a.m. ET. If you purchased the procedure before September 16, you will receive login information to participate in the live webinar. The webinar will be hosted in Streamyard.com. If you are unable to participate in the live webinar, please send us your questions in advance so that we can be sure to address your questions in the live webinar. You will be able to download the recording from the Dropbox folder after the live webinar and you can watch it as many times as needed. If you need training on how to manage you design control process, that is covered in a different training webinar.
What is included in the design control procedure?
The design controls procedure scope covers the design and development of new medical devices, including their packaging and labeling, and to modifications and upgrades of existing devices. This procedure applies from the approval of the initial design. This procedure is not applicable to research activities that precede design and development.
Please note: This product will be delivered to the email address provided in the shopping cart transaction. After the purchase is verified, please check your email for the download.
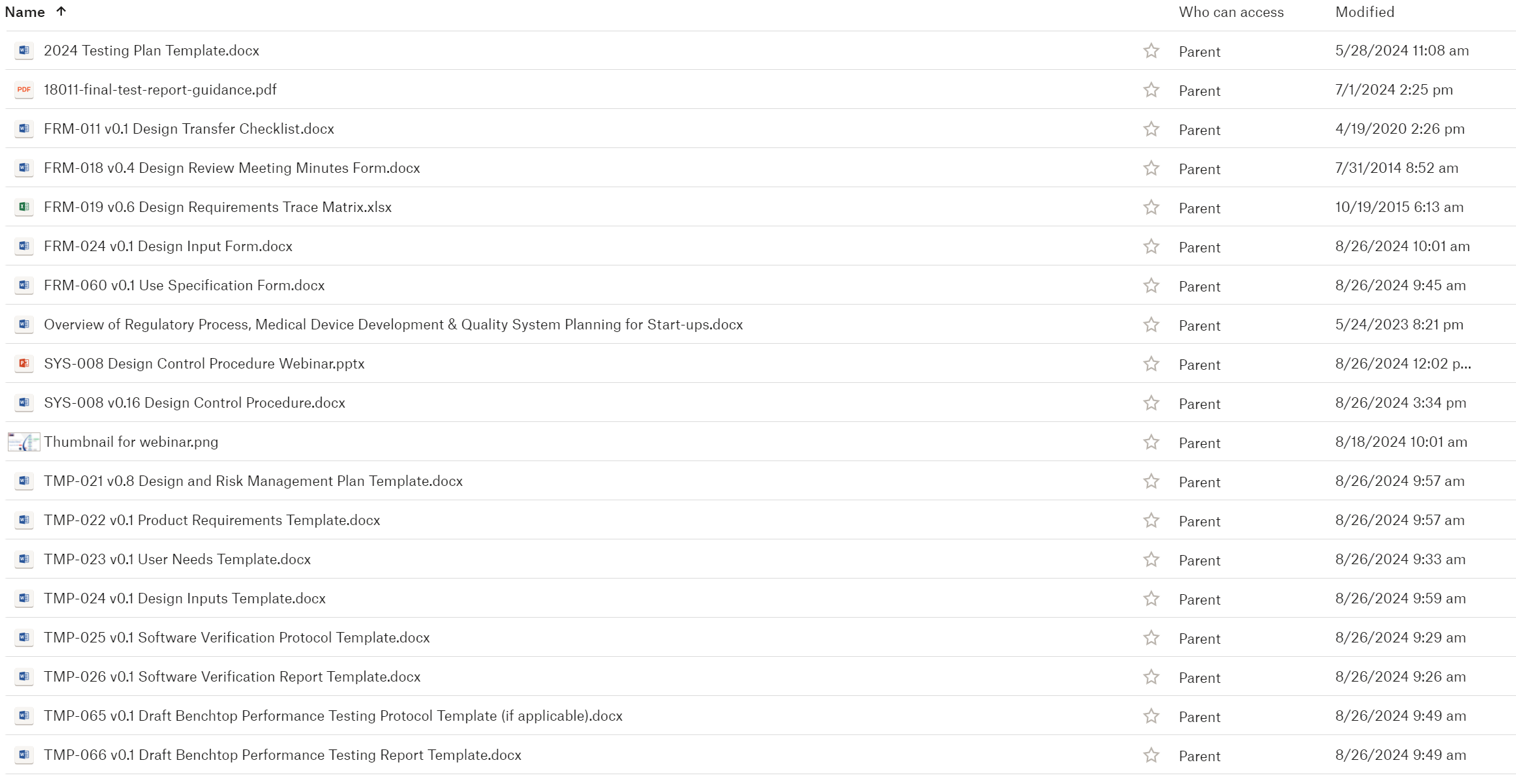
The purchase of this procedure also includes several forms and templates. The design review form is very simple to streamline your meeting documentation and simplify the process. The design requirements matrix is a fusion of two different documents used by other device companies: 1) a design FMEA, and 2) an input/output/verification/validation (IOVV) matrix. The design plan is a combined design and risk management plan. The plan combines all the requirements of: 1) a phase/gate design process; 2) the risk management activities required in ISO 14971:2019, and Annex I, sections 1-9, of Regulation (EU) 2017/745; 3) software validation documentation requirements; and 4) formative and summative usability testing requirements. The following is the full list of forms and templates provided with the SYS-008, Design Controls Procedure:
If you are interested in our design controls procedure, you might also be interested in our risk management procedure (SYS-010) and our two related training webinars:
- Design Controls Training Webinar
- ISO 14971 Risk Management Webinar- updated for ISO/DIS 14971:2018 & Regulation (EU) 2017/745
To view all available procedures, click here.
To review a sample Medical Device Academy procedure, click below:
About Your Instructor
Rob Packard is a regulatory consultant with ~25 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Rob was a senior manager at several medical device companies—including the President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009 to 2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Rob’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510k submissions. The most favorite part of his job is training others. He can be reached via phone at +1.802.281.4381 or by email. You can also follow him on YouTube, LinkedIn, or Instagram.




Our company is interested in this product. We would like to purchase the Design Control Procedures/Forms. However, we need the quotation for this product. I am not sure that your company can prepare it for us or not?
Thank you for your inquiry. I will send you a quotation in a moment. You will be able to pay directly by credit card from the emailed invoice. In the future, if you (or anyone else) would like a quotation they only need to contact Becca Taylor (billing@medicaldeviceacademy.com).
Rob