MDUFA V is the agreement between the FDA and the medical device industry to fund the review of medical device submissions by the FDA.
What is MDUFA V?
The Medical Device User Fee and Modernization Act (MDUFMA or MDUFA) is a set of agreements between the Food and Drug Administration (FDA) and the medical device industry to provide funds for the Office of Device Evaluations (ODE) to review medical device submissions. FDA user fees were first authorized via MDUFMA in 2002 for FY 2003. Each MDUFA reauthorization has lasted five years, and FY 2023 is the 21st year.
How are the MDUFA V user fees decided?
Section 738A(b)(1) of the FD&C Act requires that the FDA consult with various stakeholders, including representatives from patient and consumer advocacy groups, healthcare professionals, and scientific and academic experts, to develop recommendations for the next MDUFA five-year cycle. The FDA initiated the reauthorization process by holding a public meeting on October 27, 2020, where stakeholders and other public members could present their views on the reauthorization. The following is a list of the four industry groups represented in the MDUFA V negotiations with the FDA:
- Advanced Medical Technology Association (AdvaMed)
- Medical Device Manufacturers Association (MDMA)
- Medical Imaging & Technology Alliance (MITA)
- American Clinical Laboratory Association (ACLA)
The FD&C Act further requires that the FDA continue meeting with the representatives of patient and consumer advocacy groups at least once every month during negotiations with the regulated industry to continue discussing stakeholder views on the reauthorization and their suggestions for changes.
What are FDA user fees?
At the very core of it, the FDA user fees fund the FDA Office of Device Evaluation (ODE) budget. Without these user fees, the FDA cannot begin reviewing a medical device submission. This includes 510k, PMA, and De Novo submissions. Before the FDA assigns a reviewer to your submission, you must pay the appropriate device user fee in full unless eligible for a waiver or exemption. If you pay the user fee by credit card, you must allow a few extra days for the user fee to clear. Otherwise, your submission will be placed on “User Fee Hold.” Small businesses may qualify for a reduced fee. The FDA announced the FY 2026 FDA User Fees on July 31, 2025. The FDA will announce the user fees for FY 2027 in a Federal Register notice around August 2026.
When does MDUFA V take effect?
Our team regularly checked the announcement of the MDUFA V user fees from August until the October 5, 2022 announcement. The announcement of the FY 2023 user fees was delayed because Congress did not approve the MDUFA reauthorization until the last week of September. The new user fees were initially expected to take effect on October 1, 2022, but the announcement of actual user fees for 2022 was announced on October 5, 2022. This was two months later than expected.
Why was MDUFA V delayed, and will it happen again?
MDUFA V was delayed because the user fee reauthorization requires an act of Congress. The House of Representatives approved the Food and Drug Amendments of 2022 on June 8, 2022. However, the Senate did not file a bill until after the August recess. There were also differences between the legislation the House and the Senate proposed. Therefore, to ensure that the FDA did not have to furlough employees when MDUFA IV funding expired, the President approved and signed a temporary reauthorization on September 30, 2022. The short-term continuing resolution is a temporary stopgap to fund the FDA until December 16, 2022. However, the continuing resolution covers funding for medical device user fees through September 30, 2027. Therefore, the device industry can expect the FDA to continue to operate regardless of the outcome of temporary policies that expire this December. Still, similar delays occurred with previous MDUFA reauthorization, and we expect more of the same US partisan politics between August 2027 and the November 2027 election.
How much did MDUFA V user fees increase?
The increase is dependent upon the fee type. Annual registration fees are increasing by 14.47% (i.e., $5,672 to $6,493). The MDUFA V user fees increased by a stupendous amount (+55.90%) from $12,745 to $19,870 for the 510k user fees. Yikes! De Novo Classification Requests increased by 17.79% from $112,457 to $132,464. Other submissions increased by similar amounts. For more details, check out the table below (also posted on our homepage).

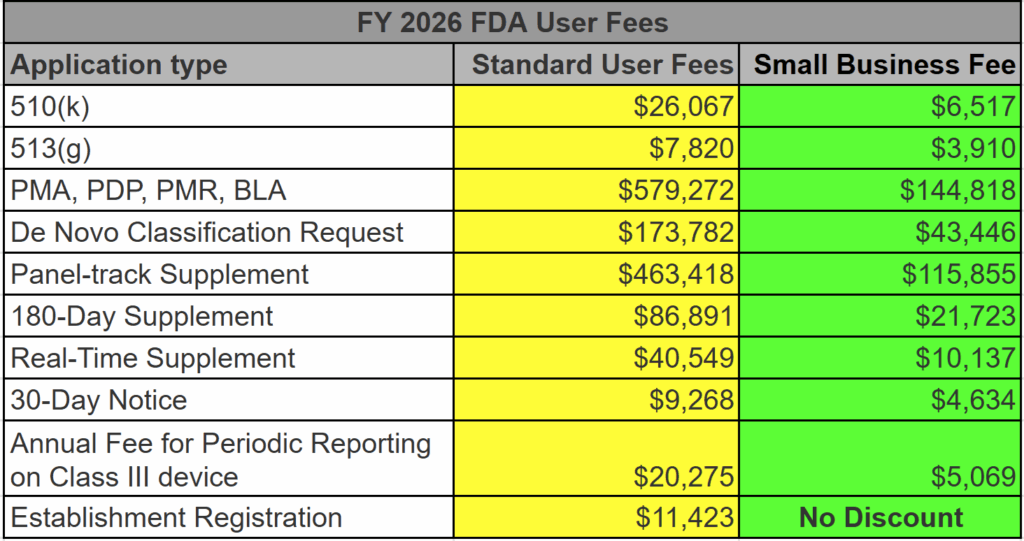
Do user fees ever decrease?
If we lived in a magical world where gas prices dropped and stayed low, the inflation-adjusted pricing would decrease for FDA user fees. That has happened once, but I fit into skinny jeans once too. The increase in FDA user fees from FY 2023 to FY 2024 was 9.5%, except the Annual Registration Fee, which increased by 17.87% to $7,653.
Why is August 1st important?
August 1st is the first day the FDA accepts Small Business Certification Requests for the new fiscal year. That means any small business that wants to keep small business status needs to reapply, and any new business that qualifies for small business status must also apply. The importance of applying for small business status is how much you could save on your submission. The FDA will complete its review of the Small Business Certification Request within 60 calendar days of receipt. Upon completion of the review by the FDA, the FDA will send you a decision letter with your small business designation number or a justification for denial.
Does small business status expire?
Yes, small business status expires. The small business status expires on September 30 of the fiscal year it is granted. A new MDUFA Small Business Certification Request must be submitted and approved each fiscal year to qualify as a small business. If you forget to reapply for small business status on August 1, you can reapply anytime during the year. Still, you will temporarily lose small business status from October 1 until the qualification is renewed. The good news is there is no fee associated with submitting a Small Business Certification Request. For more information, please visit our webpage dedicated to small business qualifications.


Pingback: What is an FDA Breakthrough Device Designation? Medical Device Academy
Pingback: FDA Pre-Submission Format and Content Requirements - Medical Device Academy Medical Device Academy
Pingback: FDA Pre-Submission Format And Content Requirements - Plato Data Intelligence
Pingback: What is the De Novo review timeline? Medical Device Academy