Medical Device Academy has updated our usability procedure (SYS-048) bundle to include new templates for use-related risk analysis (URRA).
What’s included in the usability procedure webinar bundle?
A human factors testing protocol template, training webinar, and exam are included with the procedure. These documents are updated for ISO 13485:2016 and the new European Regulations. The usability procedure meets the requirements of IEC/ISO 62366-1 (2015) and the FDA guidance on Human Factors for Medical Devices. The following is a list of items included in this bundle:
- SYS-048 A, Usability Procedure
- TMP-030 A, Summative (Validation) Usability Testing Protocol Template
- TMP-040 A, Use-Related Risk Analysis (URRA) Template
- TMP-041 A, Human Factors and Usability Report Template
- Native Slide Deck for Usability Training Webinar
- Recording of the Usability Training Webinar
What is the human factors process or usability engineering?
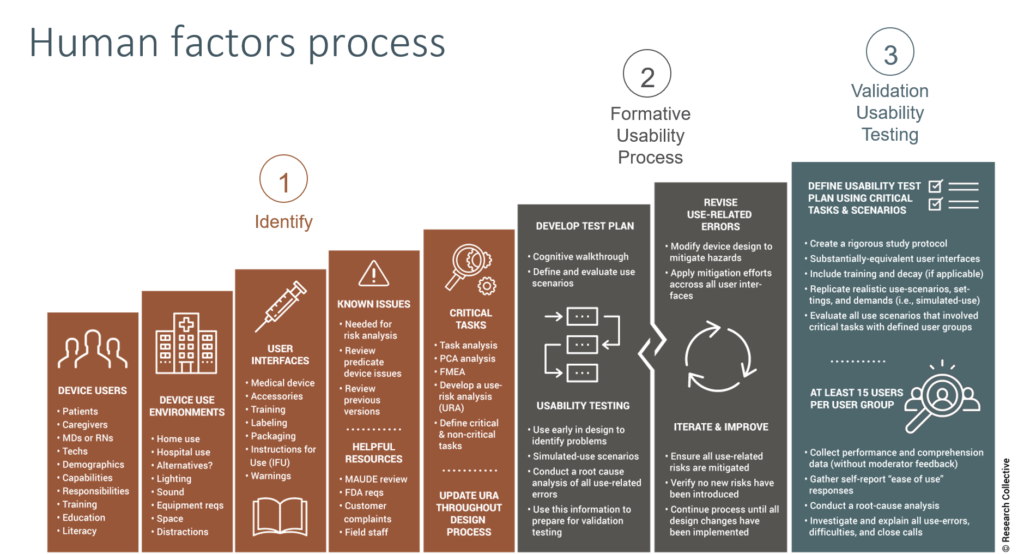
Many people struggle to understand what the human factors process or usability engineering is. In general, the human factors process is the process used for designing and developing a user interface in order to prevent use errors. It is important that design teams are trained on human factors and usability engineering. It is also important to integrate the human factors activities into the design plan or you will find that design changes are required in order to pass your summative usability testing.
Details of the Usability Procedure (SYS-048)
The Usability procedure is an 18-page procedure that is intended for any medical device design team. The procedure includes a list of definitions that are specific to usability engineering, suggested monitoring and metrics for your usability process, risk management controls to ensure that the process is effective, and a list of records with descriptions and record locations identified. The bulk of the procedure is divided into three sections: 1) planning and preparation steps, 2) planning of the summative (validation) usability testing, and 3) developing the study protocol and performing the summative usability testing. The procedure does not include extensive details on formative testing, because the nature of formative usability testing is typically less formal and it can vary greatly depending upon which usability risks are being evaluated.
When is the usability engineering / human factors webinar?
The human factors and usability engineering webinar was recorded by Rob Packard and Bryant Foster on Tuesday, September 24, 2019. The procedure and templates were updated after that webinar for compliance with IEC 62366-1 and FDA expectations for human factors testing.
What you will learn in our usability training webinar
You can also download the FDA guidance for human factors.
What Standards does the FDA Recognize for human factors?
The FDA officially recognizes device-specific and general consensus standards published by national and international standards bodies. Standards recognized by the FDA related to human factors and the application of Human Factors Engineering/Usability Engineering (HFE/UE) to medical devices can be found in a table published by the FDA in the link provided Here.
Please note: This product will be delivered to the email address provided in the shopping cart transaction. After the transaction is verified, please check your email for the download. To view all available procedures click here.
Other Usability Engineering / Human Factors Training
- Human Factors Training Series – $950
- Formative Usability Testing Webinar & Template Bundle – $79
- Use Error and Abnormal Use Decision Tree Training – $79
- Use Specification Template & Webinar Bundle – $79
- Known Use Errors Search Webinar – $129
- Task Analysis Template & Webinar Bundle – $129
- Use-Related Risk Analysis (URRA) Template & Webinar Bundle – $299
- Participant Screening & Data Collection Forms – $79
- Summative Usability Testing Protocol & Webinar Bundle – $199
- Summative Usability Testing Report – Live-streaming Free Webinar

