GSPRs – General Safety and Performance Requirements
GSPRs are the General Safety and Performance Requirements for CE Marking of medical devices and IVDs in Annex I of the EU MDR and IVDR.
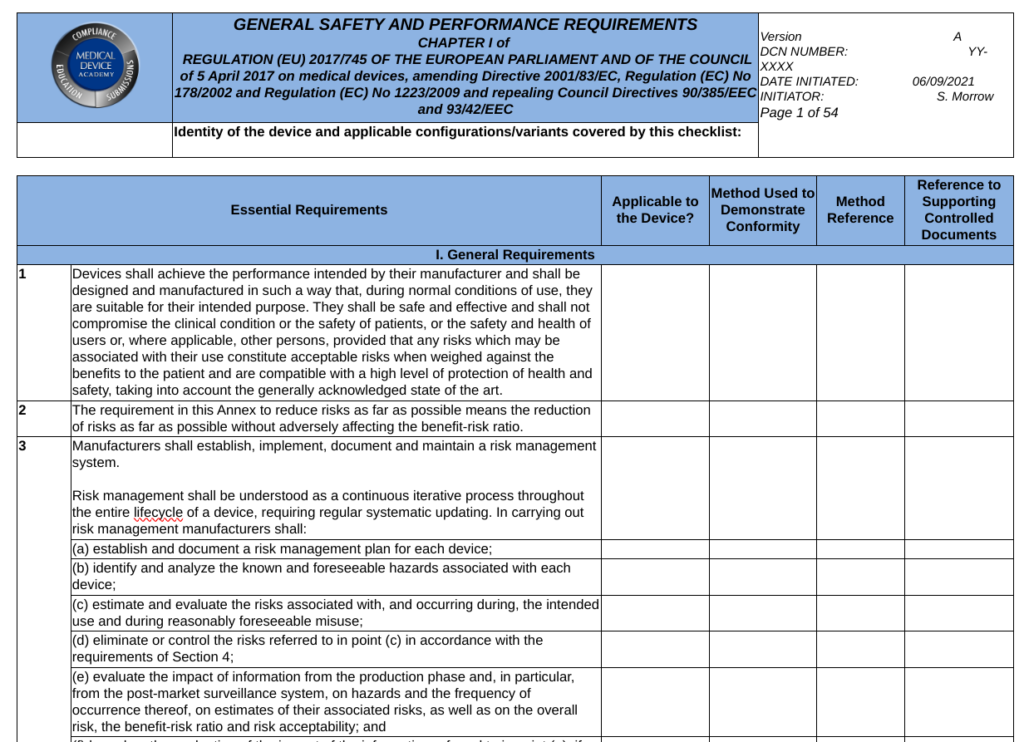
What are the GSPRs?
General Safety and Performance Requirements (GSPRs) are the requirements for safety and performance specified in Annex I of the EU MDR and EU IVDR. GSPRs are divided into Chapter I (i.e., – Sections 1-9 of the MDR and Sections 1-8 of the IVDR are the General requirements), Chapter II (i.e., – Sections 10-22 of the MDR and Sections 9-19 of the IVD are the Requirements regarding design and manufacture), and Chapter III (i.e., Section 23 of the MDR and Section 20 of the IVDR are the requirements regarding the information supplied with the device or IVD). All devices must meet the requirement of Chapter I and Chapter III, but the applicability of Chapter II depends upon the technological characteristics of the device or IVD.
Where do the GSPRs go in your technical documentation?
When a Notified Body reviews your technical documentation, they expect you to provide either a complete technical file or technical file index that is organized in accordance with Annex II of the MDR or IVDR. Section 4 of Annex II is labeled “General Safety and Performance Requirements.” This section is where your GSPR checklist should be located in the technical file or technical file index. Generally a GSPR checklist is considered the best way to document these requirements. The checklist should should provided traceability to each specific requirement and the following elements:
- the applicability or non-applicability of each requirement; justifications shall be documented for non-applicability
- the method or methods used to demonstrate conformity with each requirement
- the harmonized standards (i.e., EN standard) and/or Common Specifications (CS) applied
- identification of controlled documents that provide evidence of conformity with the harmonized standards or CS
What are the subparts of each chapter in the GSPRs?
Chapter I
The general requirements for safety and performance (i.e., Chapter I in Section 1-9 of the MDR and Section 1-8 of the IVDR) are primarily focused on risk management requirements. These first few sections state that the manufacturer must ensure that the device or IVD is safe, effective, and does not compromise the clinical condition or safety of patients or users. The manufacturer must take into account the generally acknowledged state of the art. Risks must be reduced as far as possible without adversely affecting the benefit-risk ratio. The manufacturer must implement a risk management system. Risks associated with use errors shall be eliminated or reduced as far as possible. The characteristics of performance shall not be adversely affected the conditions of use, transport and storage during the lifetime of the device or IVD. Finally, all residual risks shall be minimized and be acceptable when weighted against the benefits to the patients and/or user arising from the intended use during normal conditions of use.
Chapter II of the MDR
This Chapter of the GSPRs is organized into the following subsections of the MDR:
- 10 – Performance characteristics
- 11 – Chemical, physical and biological properties
- 12 – Infection and microbial contamination
- 13 – Devices incorporating materials of biological origin
- 14 – Construction of devices and interaction with their environment
- 15 – Devices with a diagnostic or measuring function
- 16 – Protection against radiation
- 17 – Electronic programmable systems
- 18 – Active devices and devices connected to them
- 19 – Particular requirements for active implantable devices
- 20 – Protection against mechanical and thermal risks
- 21 – Protection against the risks posed to the patient or user by devices supplying energy or substances
- 22 – Protection against the risks posed by medical devices intended by the manufacturer for use by lay persons
Chapter II of the IVDR
This Chapter of the GSPRs is organized into the following subsections of the IVDR:
- 9 – Performance characteristics
- 10 – Chemical, physical and biological properties
- 11 – Infection and microbial contamination
- 12 – Devices incorporating materials of biological origin
- 13 – Construction of devices and interaction with their environment
- 14 – Devices with a measuring function
- 15 – Protection against radiation
- 16 – Electronic programmable systems
- 17 – Devices connected to or equipped with an energy source
- 18 – Protection against mechanical and thermal risks
- 19 – Protection against the risks posed by devices intended for self-testing or near-patient testing
Chapter III
Chapter III is divided into four subparts. Section 23.1 of the MDR and section 20.1 of the IVDR are the general requirements for information provided by the manufacturer (i.e., labeling). The recommended harmonized standard is EN ISO 20417:2021. Section 23.2 of the MDR and section 20.2 of the IVDR include the labeling requirements. Section 23.3 of the MDR and section 20.3 of the IVDR include requirements for information on the packaging which maintains the sterile condition of a device or IVD (i.e., label on the sterile barrier packaging). Finally, Section 23.4 of the MDR and section 20.4 of the IVDR include requirements for the Instructions for Use (i.e., IFU, Directions for Use, or User Manual).
Completing your checklist
Completing the GSPR Checklist would be easy if there were only 20-23 requirements, but most of the requirements have multiple requirements. For example, GSPR 14 of the MDR has 7 subparts, 18 of the MDR has 8 subparts, and labeling requirements are six pages long. Each subpart must be addressed when you complete the columns of the checklist. If any of the parts or subparts do not apply to your device, you need to provide a justification. When you write your justification for the non-applicability of a GSPR, you need to be careful to provide a justification for each subpart of the requirement–even if the subpart is not separately identified by a letter or number.
Download our Checklist
If you need a template for creating your own GSPR checklist, you can download our template by filling in the form below:
How do you address differences from the Essential Principles of Safety and Performance?
Health Canada also identifies Essential Principles for Safety and Effectiveness in Sections 10-20 of the Canadian Medical Device Regulations (i.e., SOR 98/282) that is similar to the European GSPRs, and Australia has a similar Essential Principles Checklist document with only a few minor differences. The Global Harmonized Task Force (GHTF) created an earlier version in 2005, but the International Medical Device Regulators Forum (IMDRF) released a newer version in 2024. Health Canada will typically accept your GSPR checklist developed for CE Marking, but a gap analysis should be performed against the Australian Regulations.
GSPRs – General Safety and Performance Requirements Read More »