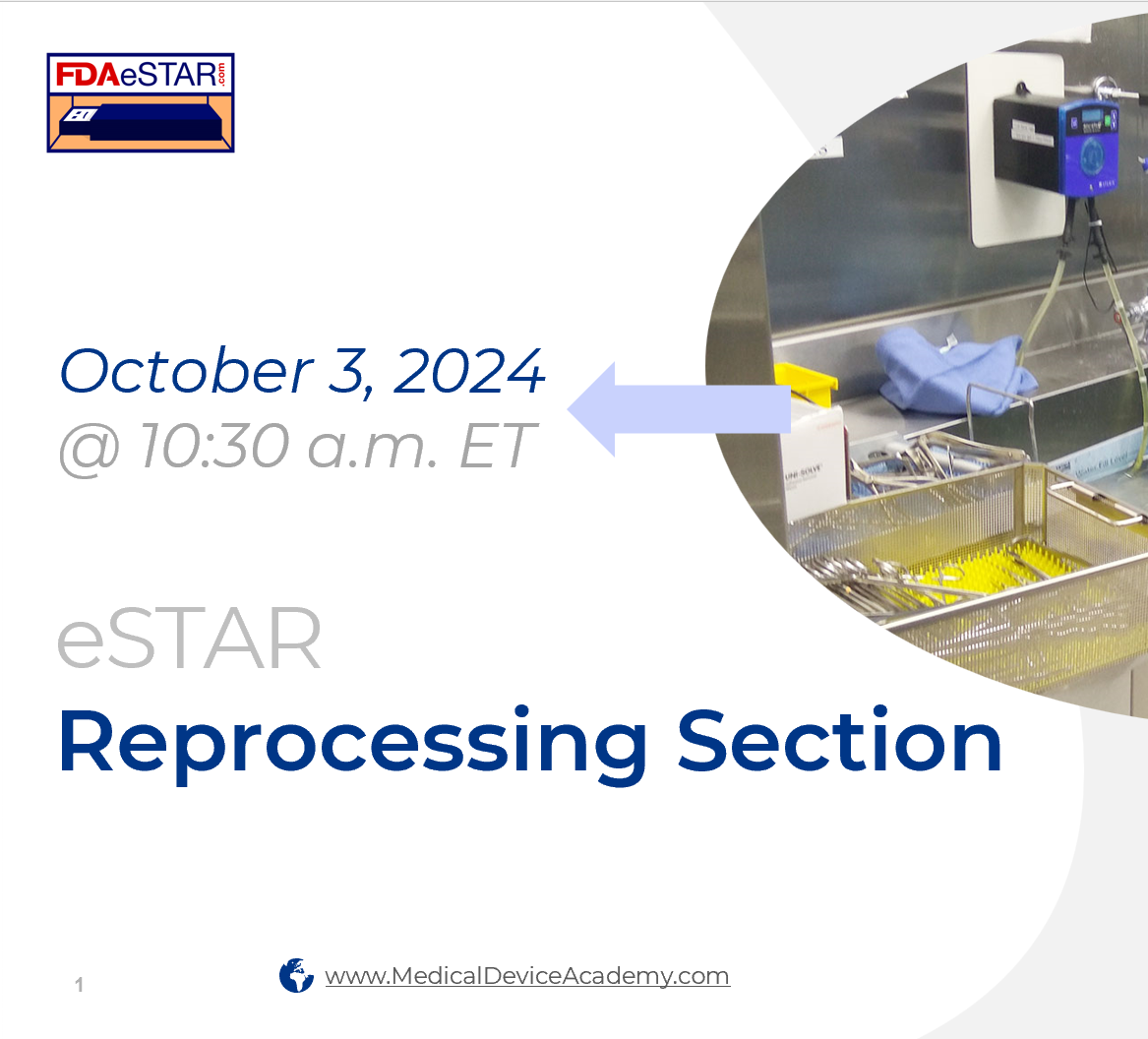
In this FDA eSTAR webinar, you will learn how to prepare the verification and validation testing required for reprocessing devices.
Reprocessing devices webinar – $79
When is the reprocessing devices webinar scheduled?
This webinar was recorded on Saturday, October 5, 2024. You can purchase it on-demand and watch the training as often as you wish.
Why should you consider registering for the webinar on reprocessing devices?
The reprocessing section of the submission is not just a paragraph referencing the recognized standards that were applied. Reprocessing devices requires design verification testing, usually costing tens of thousands of dollars. The critical factors that determine the cost of validating your reprocessing steps are:
- types of “soil” being simulated
- cleaning methods (manual, automated washing, or both)
- level of disinfection (none, low, moderate, or high-level disinfection)
- methods used to verify the effectiveness of cleaning and disinfection
- sterilization method (none, steam, EO, or VHP)
- the number of cycles being validated
All reprocessed devices require cleaning instructions to be included in the user manual or instructions for use, but those cleaning methods need to be validated. If disinfection is required, then the level of disinfection needs to be justified and validated. Finally, the sterilization method(s) need to be validated.
Hospitals that purchase your device will make sure that the methods prescribed in your instructions align with the capabilities of their sterile processing department. Therefore, most companies want to validate their device for multiple cleaning, disinfection, and sterilization methods. However, every method you validate will increase your up-front costs. For this reason, most companies limit their validation to the most widely used methods.
Another consideration is whether your methods of reprocessing devices require human factors validation. Companies usually copy standardized methods from a competitor’s instruction for use or another reprocessed device in their portfolio. Unfortunately, you never know where those instructions came from and if they were validated. Therefore, the best practice is to work with an institution that teaches sterile processing. The instructor will be able to identify problematic aspects of your device design and recommend instructions for reprocessing. Once you finalize your instructions, the same instructors can help you with summative usability testing for reprocessing your device.
What you will receive for $79
- a recording of the webinar you can replay anytime
- the native slide deck for the reprocessing devices webinar
- a copy of the FDA guidance for reprocessing medical devices
The presentation includes 27 slides and was ~35 minutes long. All content deliveries will be sent via AWeber emails to confirmed subscribers.
Q&A
Please email me questions at rob@fdaestar.com regarding the reprocessing devices webinar.
Additional Resources for 510k submissions
If you would like additional training on 510k submissions or you would like to access Medical Device Academy’s templates, you can purchase all of our templates and 510k webinars on our 510k course webpage.
About Your Instructor
Rob Packard is a regulatory consultant with ~25 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Rob was a senior manager at several medical device companies—including the President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009 to 2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Rob’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510(k) submissions. The most favorite part of his job is training others. He can be reached via phone at +1.802.258.1881 or by email. You can also follow him on YouTube, LinkedIn, or Twitter.