This article uses a case study example to explain how to determine the correct regulatory pathway for your medical device through the US FDA.

Every consultant likes to answer this type of question with the answer, “It depends.” Well, of course, it depends. If there was only one answer, you could google that question, and you wouldn’t need to pay a regulatory consultant to answer the question. A more useful response is to start by asking five qualifying questions:
- Does your product meet the definition of a device?
- What is the intended purpose of your product?
- How many people in the USA need your product annually?
- Is there a similar product already on the market?
- What are the risks associated with your product?
The first question is important because some products are not regulated as medical devices. If your product does not diagnose, treat, or monitor a medical condition, then your product may not be a device. For example, the product might be considered a general wellness product or clinical decision support software. In addition, some products have a systemic mode of action, and these products are typically categorized as a drug rather than a device–even if the product includes a needle and syringe.
The intended purpose of a product is the primary method used by the US FDA to determine how a product is regulated. This also determines which group within the FDA is responsible for reviewing a submission for your product. The US regulations use the term “intended use” of a device, but the decision is based upon the “indications for use” which are more specific. To understand the difference, we created a video explaining the difference.
Even regulatory consultants sometimes forget to ask how many people need your product annually, but population size determines the regulatory pathway. Any intended patient population less than 8,000 patients annually in the USA is eligible for a humanitarian device exemption with a special regulatory pathway and pricing constraints. If your product is intended for a population of <8,000 people annually, your device could qualify for a humanitarian device exemption, and the market is small enough that there may not be any similar products on the market.
If similar products are already on the US market, determining the regulatory pathway is much easier. We can look up the competitor product(s) in the FDA’s registration and listing database. In most cases, you must follow the same pathway your competitors took, and the FDA database will tell us your regulatory pathway.
If all of the products on the US market have different indications for use, or the technological characteristics of your product are different from other devices, then you need to categorize your product’s risks. For low-risk devices, general controls may be adequate. For medium-risk devices, special controls are required by the FDA. For the highest-risk devices, the FDA usually requires a clinical study, a panel review of your clinical data, and the FDA requires pre-market approval.
This article will use the example of bipolar forceps used with an electrosurgical generator as a case study.

What is the US FDA regulatory pathway for your device?
The generic term used for regulator authorization is “approval,” but the US FDA reserves this term for Class 3 devices with a Premarket Approval (PMA) submission. The reason for this is that only these submissions include a panel review of clinical data to support the safety and effectiveness of the device. Approval is limited to ~30 devices each year, and approximately 1,000 devices have been approved through the PMA process since 1976 when the US FDA first began regulating medical devices.
Most Class 2 devices are submitted to the FDA as Premarket Notifications or 510k submissions. This process is referred to as “510k clearance,” because clinical data is usually not required with this submission and there is no panel review of safety and effectiveness data. A 510k was originally planned as a rare pathway that would only be used by devices that are copies of other devices that are already sold on the market. However, the 510k pathway became the defacto regulatory pathway for 95+% of devices that are sold in the USA.
For moderate and high-risk devices that are intended for rare patient populations (i.e., <8,000 patients per year in the USA), the humanitarian device exemption process is the regulatory pathway.
Class 1 devices typically do not require a 510k submission, most of these devices are exempt from design controls, and some are exempt from quality system requirements. These devices still require listing on the FDA registration and listing database, but there is no review of the device by the FDA to ensure you have correctly classified and labeled Class 1 devices.
How do you find a predicate for your 510k submission?
As stated above, one of the most critical questions is, “Is there a similar product already on the market?” For our example of bipolar forceps, the answer is “yes.” There are approximately 169 bipolar forceps that have been 510k cleared by the FDA since 1976. If you are developing new bipolar forceps, you must prepare a 510k submission. The first step of this process is to verify that a 510k submission is the correct pathway and to find a suitable competitor product to use as a “predicate” device. A predicate device is a device that meets each of the following criteria:
- it is legally marketing in the USA
- it has indications for use that are equivalent to your device
- the technological characteristics are equivalent to your device
There are two search strategies we use to verify the product classification of a new device and to find a suitable predicate device. The first strategy is to use the free, public databases provided by the FDA. Ideally, you instantly think of a direct competitor that sells bipolar forceps for electrosurgery in the USA (e.g., Conmed bipolar forceps). You can use the registration and listing database to find a suitable predicate in this situation. First, you type “Conmed” into the database search tool for the name of the company, and then you type “bipolar forceps” in the data search tool for the name of the device.
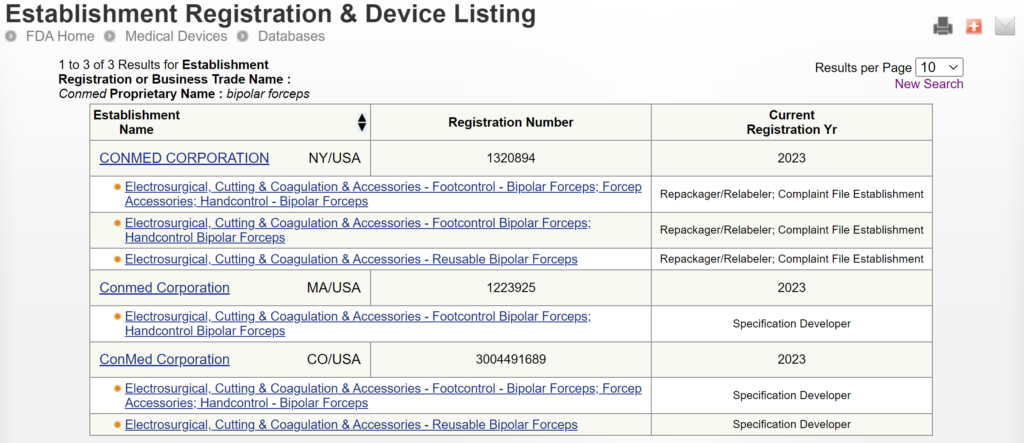
If you are unaware of any competitor products, you will need to search using the product classification database instead. Unfortunately, this approach will result in no results if you use the terms “bipolar” or “forceps.” Therefore, you will need to be more creative and use the word “electrosurgical,” which describes a larger product classification that includes both monopolar and bipolar surgical devices that have many sizes and shapes–including bipolar forceps. The correct product classification is seventh out of 31 search results.
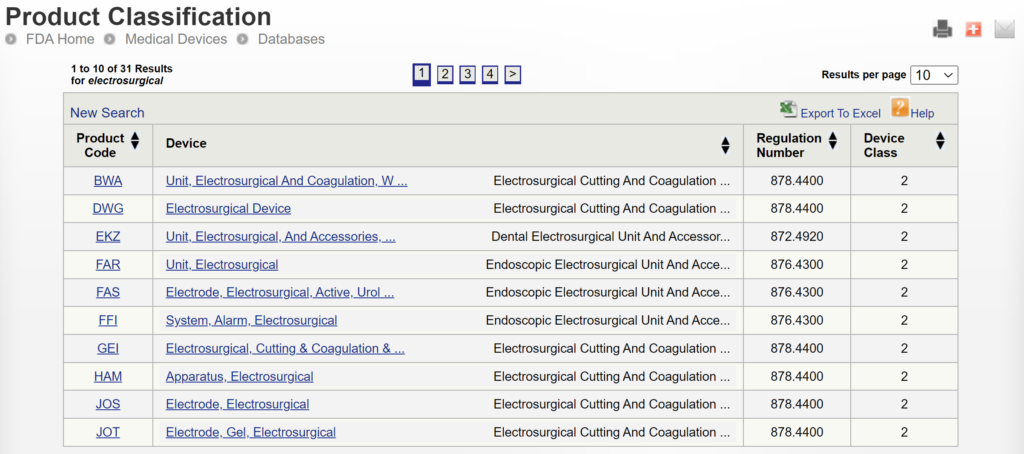
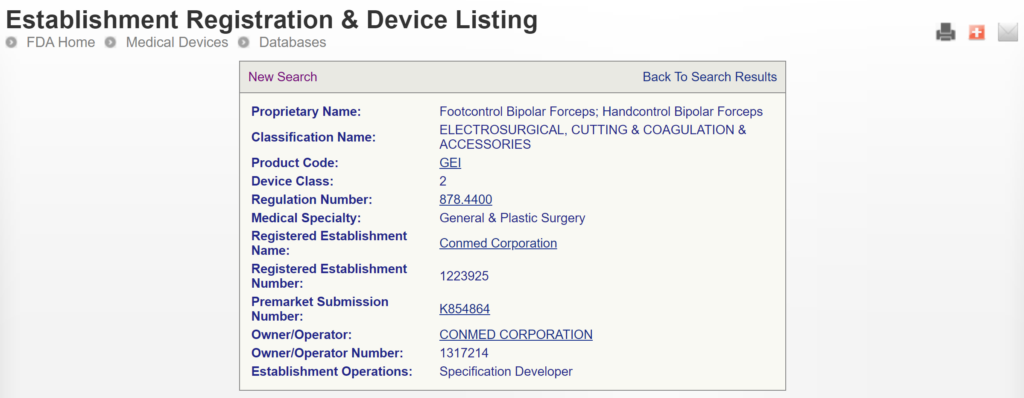
The most significant disadvantage of the FDA databases is that you can only search each database separately. The search is also a boolean-type search rather than using natural language algorithms that we all take for granted. The second strategy is to use a licensed database (e.g., Basil Systems).
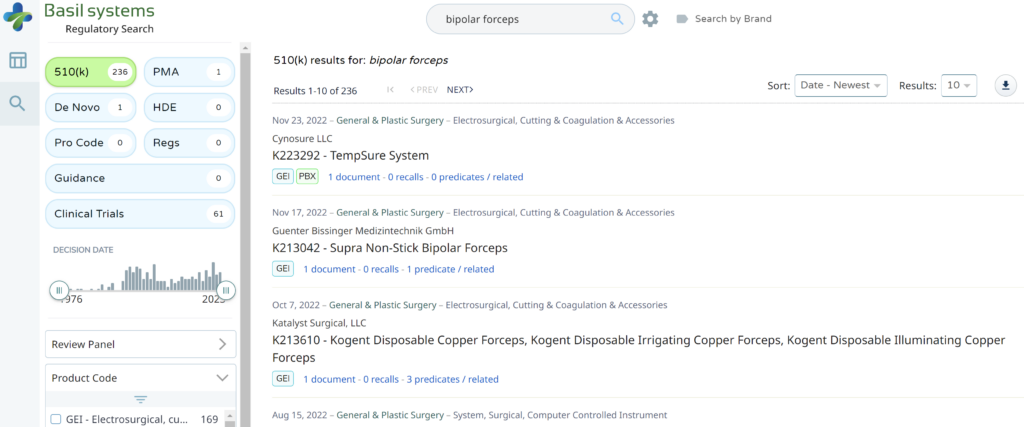
Searching these databases is more efficient, and the software will provide additional information that the FDA website does not offer, such as a predicate tree, review time, and models listed under each 510k number are provided below:
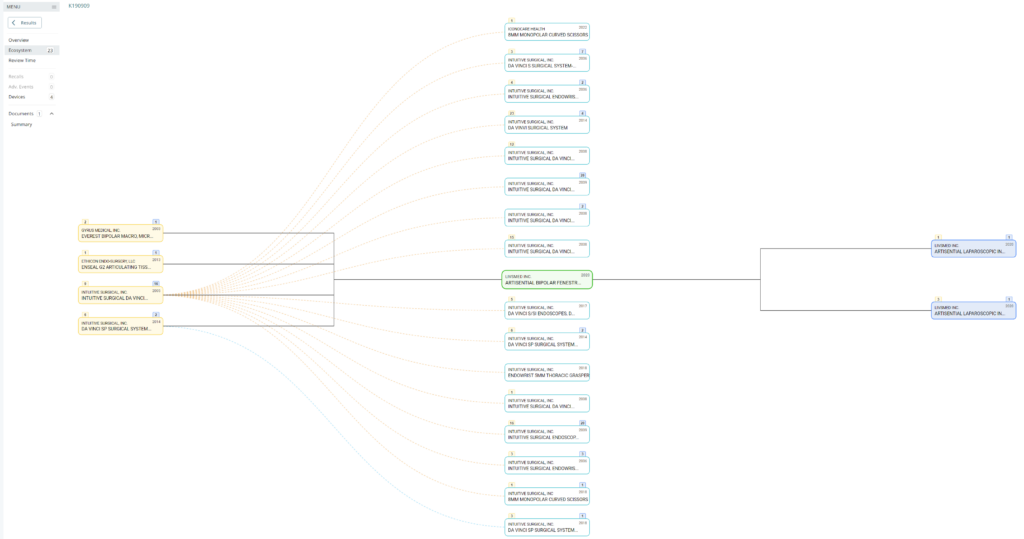
What does the predicate tree look like for the predicate device you selected?
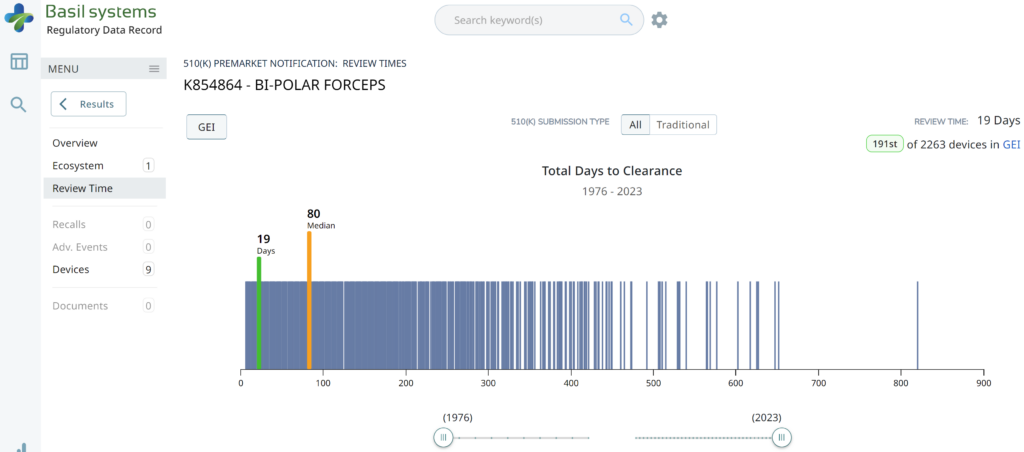
I’m glad I don’t need to manually enter the 510k review time for 2,263 devices to create the above graph.
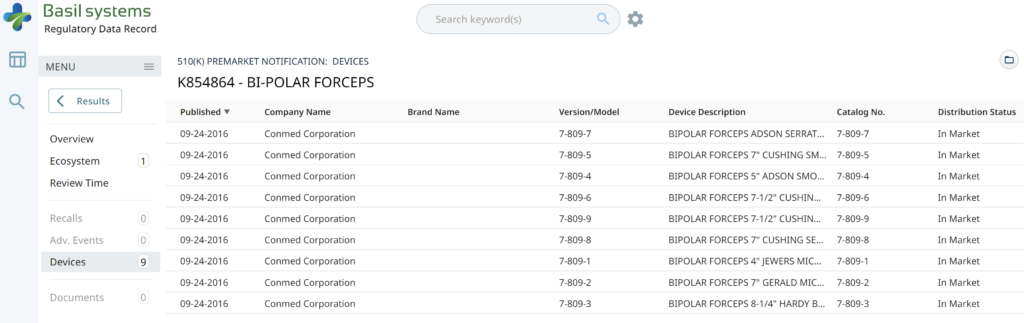
Wouldn’t having the model numbers for every device identified in the US FDA listing database be nice?
Another advantage of the Basil Systems software is that the database is lightning-fast, while the FDA is a free government database (i.e., not quite as fast).
How do you create a regulatory pathway strategy for medical devices?
The best strategy for obtaining 510k clearance is to select a predicate device with the same indications for use that you want and was recently cleared by the FDA. Therefore, you will need to review FDA Form 3881 for each of the potential predicate devices you find for your device. In the case of the bipolar forceps, there are 169 devices to choose from, but FDA Form 3881 is not available for 100% of those devices because the FDA database only displays FDA Form 3881 and the 510(k) Summary for devices cleared since 1996. Therefore, you should select a device cleared by the FDA in the past ten years unless there are no equivalent devices with a recent clearance.

In addition to identifying the correct product classification code for your device and selecting a predicate device, you will also need to develop a testing plan for the verification and validation of your device. For electrosurgical devices, there is an FDA special controls guidance that defines the testing requirements and the content required for a 510k submission. Once you develop a testing plan, you should confirm that the FDA agrees with your regulatory strategy and testing plan in a pre-submission meeting.
Which type of 510k submission is required for your device?
There are three types of 510k submissions:
- Special 510k – 30-day review target timeline
- Abbreviated 510k – 90-day review target timeline (requires summary reports and use of recognized consensus standards)
- Traditional 510k – 90-day review target timeline
The special 510k pathway is intended for minor device modifications from the predicate device. However, this pathway is only eligible to your company if your company also submitted the predicate device. Originally it was only permitted to submit a Special 510k for modifications that require the review of one functional area. However, the FDA recently completed a pilot study evaluating if more than one functional area could be reviewed. The FDA determined that up to three functional areas could be reviewed. However, the FDA decides whether they can complete the review within 30 days or if you need to convert your Special 510k submission to a Traditional submission. Therefore, you should also discuss the submission type with the FDA in a pre-submission meeting if you are unsure whether the device modifications will allow the FDA to complete the review in 30 days.
In 2019 the FDA updated the guidance document for Abbreviated 510k submissions. However, this pathway requires that the manufacturer use recognized consensus standards for the testing, and the manufacturer must provide a summary document for each test report. The theory is that abbreviated reports require less time for the FDA to review than full test reports. However, if you do not provide sufficient information in the summary document, the FDA will place your submission on hold and request additional information. This happens for nearly 100% of abbreviated 510k submissions. Therefore, there is no clear benefit for manufacturers to take the time to write a summary for each report in the 510k submission. This also explains why less than 2% of submissions were abbreviated type in 2022.
The traditional type of 510k is the most common type of 510k submission used by manufacturers, and this is the type we recommend for all new device manufacturers.
