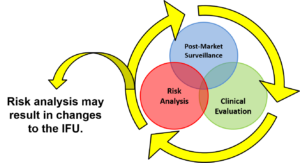
This IFU validation webinar describes how to perform IFU validation prior to commercialization and how to conduct post-market surveillance to ensure that your instructions for use continue to be suitable as your user population and patient population expand.