This article discusses the need to requalify EO sterilization validation and explains what is included in our EO sterilization procedure.
Do you need an EO sterilization procedure?
ISO 11135-1 is the international standard for sterilization validation for Ethylene Oxide (EO or EtO) sterilizers. The standard describes multiple methods of sterilization validation: 1) overkill approach, 2) single lot release, and 3) parametric release. The overkill approach is the most common method for validation of your EO sterilization process. The overkill approach is the method recommended by Medical Device Academy’s EO sterilization procedure. If you use a contract sterilizer, the sterilizer will already have completed an Installation Qualification (IQ) and an Operational Qualification (OQ). You must complete a Performance Qualification (PQ) for your product. A typical PQ for initial process validation consists of the following:
- Process Challenge Device (PCD) validation
- Bioburden measurement
- EO residual measurement (as per ISO 10993-7)
- Fractional Cycle (at least one)
- 3 Half Cycles
- 3 Full Cycles (or 1 Full Cycle, if performed in parallel with the three half-cycles)
Purchase the EO Sterilization Validation Procedure (SYS-031) – $299
What do MPQ and PPQ mean?
In the ISO 11135 standard, steps #4 and #5 listed above are referred to as the microbial performance qualification (MPQ), while #6 is the physical performance qualification (PPQ). For a successful MPQ, some PCDs must be non-sterile after a fractional cycle to demonstrate the ability to recover the BI challenge organism. After a half-cycle, however, all biological indicators should be sterile.
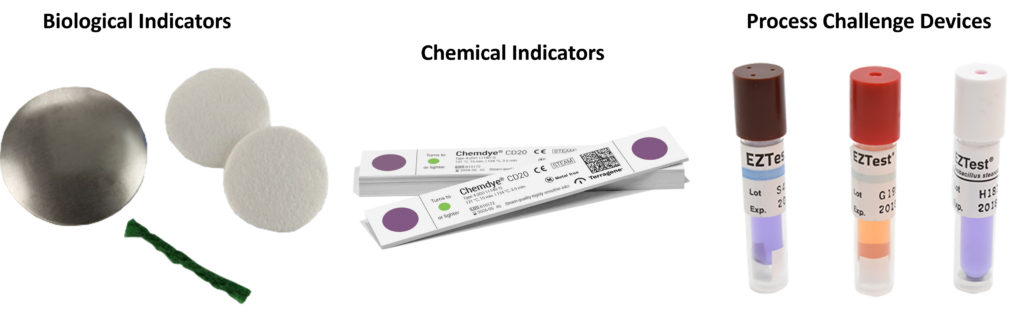
What are BIs, CIs, and PCDs?
To avoid destructive testing, EO sterilization processes verify sterility by using process challenge devices (PCDs) located outside the device’s primary and secondary packaging. PCDs are more challenging to sterilize than the native bioburden on your device, and the PCDs can be quickly removed from a sterilized pallet without disturbing the wrapping of the pallet. PCDs are also referred to as external biological indicators (BIs). Biological indicators are used internally and externally to your primary sterile barrier packaging during the EO sterilization validation process, but only external BIs are used during routine EO sterilization. You can create your own customized PCD for devices that are especially difficult to EO sterilize (e.g., stopcocks), but you must verify that the PCDs are more challenging to kill than an internal BI in a fractional cycle. Commercially available PCDs often incorporate a chemical indicator (CI) into the label or the cap of the PCD, and some are incorporated into sophisticated tracking software with automatic incubators that read a barcode on the PCD label and detect the results of incubating the BIs in media. These systems are rapid, self-contained BIs that provide validated results in hours, minutes, or seconds.
Outsourcing EO sterilization and requalification
Ethylene oxide sterilization is usually outsourced to a contract sterilizer due to the environmental and safety requirements of working with EO. The contract sterilizer will provide a generic protocol for full validation that is compliant with ISO 11135-1. However, the ISO 11135-1 standard requires that manufacturers perform annual process reviews to evaluate the need to requalify/re-validate the sterilization process. Assuming there have been no problems or changes to the product or EO sterilization process, re-validation is not required at the end of the first year. However, companies are required to re-validate the process after two years–even if there have been no changes.
Longer frequencies for requalification cycles
If there have been no changes to the sterilization process, the product, or the biological indicators, then the manufacturer can use this as a justification for waiting until two years have elapsed before re-validating the ethylene oxide sterilization process. Also, there should be no evidence of sterilization failures or other problems with the validated process. However, that alone is not necessarily enough to justify extending the duration between validations beyond two years. Companies that justify intervals of three or more years have multiple products that use the same EO sterilization process.
In this case, the manufacturer may alternate annually between three, four, or even five different product families that are using the same EO sterilization process. In this case, one of the product families is being re-validated each year or every two years, but the interval between validations for any one product family is longer. This approach is valid if the products are made of similar materials and use the same EO sterilization process. If you only have one product, then you need to re-validate the sterilization process once every two years to verify the process remains active.
Minimum revalidation requirements
When you determine that it is time to re-validate your ethylene oxide sterilization process, you need to perform the following tests to meet the minimum requirements of ISO 11135-1:
- Re-validation of PCD
- Bioburden measurement
- EO residual measurement
- 1 Half Cycle
- 1 Full cycle (to verify the EO residuals are acceptable)
The purpose of #1 is to verify that the resistance of internal BIs used in the half-cycle is more resistant than the product bioburden. The purpose of #2 is to verify that bioburden levels have not changed, and the type of organisms has not changed. In practice, most companies monitor bioburden quarterly, and therefore this step should be routine. Step 3, EO residual measurement, should be performed to verify that there have not been minor changes to the product or process that would increase the concentration of EO, Ethylene Chlorohydrin (ECH), or Ethylene Glycol (EG) beyond the Tolerable Contact Limit (TCL). The purpose of this third test is to prevent localized irritation caused by residual chemicals from the ethylene oxide sterilization process.
Step 4 of the re-validation is intended to verify that a full injection of EO is more than required to kill the bioburden present for the number of injections required for a half-cycle.
The final step is to perform a full cycle. The product from the full cycle is typically used for EO residual testing. Any product from the full cycle that is not used for testing can be sold after sterility testing is complete.
Partial loads & rework
If you occasionally sterilize loads that are less than “full loads,” then you need to ensure that you have validated a minimum load or a specific partial load (e.g., half-pallet, instead of a full pallet). In the case of a partial or minimum load, you may identify different locations in your load that is considered “worst-case.” These are the locations that had PCDs that were not sterile in a fractional cycle.
Most companies do not have concerns about the cost of the actual sterilization runs during re-validation, and biological indicators are typically less expensive than boxes of products. The primary cost concern for re-validation is any product that must be scrapped. Therefore, many companies will accumulate dunnage (i.e., empty packaging or scrap product) over time to fill a sterilizer. This dunnage may be used to ensure that every load is full, or it may only be used for re-validation.
Another alternative to using dunnage for re-validation is to validate a rework process. Any product exposed to a fractional or half-cycle can be re-sterilized in a full cycle. To justify the commercial use of that product, a company needs to validate that the product will not be damaged by exposure to two full cycles. One of the key acceptance criteria for rework is the EO residual levels in the product. However, the manufacturer also needs to determine if any product deterioration by a second exposure to EO would affect performance.
Other EO sterilization considerations
Many companies do a poor job of reviewing the potential impact of changes to a product, packaging, and biological indicators. Ideally, initial validation involves different lots of product, packaging, and biological indicators to assess lot-to-lot variability. However, the packaging and biological indicators often consist of only one lot during validation. Minor changes to the tolerances may reduce the amount of ethylene oxide that is absorbed by the product or change the resistance of the biological indicator to the sterilization process. Therefore, these minor changes should trigger a re-validation.
Changes in suppliers with the same specification can also be difficult to evaluate. If a component is made of a material that absorbs EO, then it may be recommended to re-validate sterilization for any changes to suppliers of those components. Re-validation in these cases may consist of only a fractional cycle, half cycle, or full cycle to evaluate risks associated with the change.
Who should evaluate the need for EO sterilization requalification?
Evaluating the need for re-validation should include inputting three types: 1) microbiological, 2) materials, and 3) performance. To make these assessments, typically, a cross-functional team is needed. Someone responsible for design and development can assess the performance impact of changes. A materials engineer is generally needed to assess the interaction between components and EO. Finally, a microbiologist is needed to confirm that there is no impact related to biological indicators or bioburden.
 Additional Sterilization Training
Additional Sterilization Training
Medical Device Academy has two webinar recordings related to sterilization validation:
- Bioburden Failure Analysis (March 27, 2015)
- Sterilization Validation Webinar (October 10, 2024)
If you need assistance with sterilization validation or bioburden failure analysis, please schedule a call with us by visiting our Contact Us page.



A very good article. It is very important to conduct a re-validation whenever a product packaging is modified or changed. You have made it very clear in this article.
Excellent Article pertaining to need of the re-validation of ETO Sterilizer process, especially routine measurement of bio burden measurement instead of quarterly done.
When calculate the TCL for a permanent device, you should use the results of the exhaustive (24 hrs extraction) or you can use an extra test results with 8 hrs extraction?
Thanks!
Hi! I would like to know references to below statement.
“Any product that is exposed to a fractional cycle or half-cycle can be resterilized in a full cycle. In order to justify the commercial use of that product, a company needs to validate that the product will not be damaged by exposure to two full cycles.”
In the case of someone need to repeat the sterilization, do we need to validate up to 3x exposure to full cuycles.
thanks
Thank you for your question Roslan. There is no “reference” for that statement. It is a scientific rationale: the risk of exposure to the extra fractional or half-cycle is no worse than the exposure to two (2) full cycles. Therefore, if you validate the product is not damaged by exposure to two (2) full cycles, then the product should also be undamaged by 1.5 cycles. The other advantage of validating two (2) full cycles, is that you can re-sterilize product if necessary. Validating a 3x exposure is not needed.
Good article! Where did you find the requirement of performing EO residual studies in re-qualification (or “re-validation”)?
Best wishes
Manuel
Great question Manuel. When you perform your annual review of the process, if there is a shift in products sterilized, this can impact the absorption of EO gas. If there is no change in the products sterilized, then this would not need to be repeated.
Rob
Helpful article! I just had a quick question. Is there a reference to this statement, “However, companies are expected to re-validate the process after two years–even if there have been no changes.”?
Yes, but the re-validation is abbreviated, and the standard explains the rationale behind the need for re-validation. Essentially, the biggest risk is a change in bioburden fluora that you are trying to kill due to the selective pressure applied by using cleaning and disinfection methods in the production areas over months and years. There are also potential unseen changes in the contribution by your suppliers and their facilities.
Dear Sir,
I would like to know about the re validation of the Eto sterilization.
We did our Knee implant (UHMPWE ) with Eto sterilization at another center so my question is that do we need to revalidate the Eto sterilization or the sterilization center do the Revalidation?
Could you please clarify this doubt.
Thank you.
Yes, you need to provide a scientific justification for why revalidation is not required or revalidate. If both locations were owned by the same company (e.g. Sterigenics), then they may be able to help with a scientific justification. However, if the facilities are different owners it is almost impossible to provide a justification for no revalidation. Even small differences in the chamber will change the effectiveness of the sterilization process and affect where BIs need to be located and how product will be loaded into the chamber.
Pingback: How much does a 510k cost? - Medical Device Academy Medical Device Academy
Pingback: Performance Qualification (PQ) for EO Sterilization Validation - Medical Device Academy Medical Device Academy
Pingback: How to select and help validate the best sterilization method? Medical Device Academy
Hi, could you help clarify if we use one vendor (intermediate vendor) who contracts EtO to another vendor, would the intermediate vendor be responsible for maintaining any annual revalidation review documents? Or would this solely rely on the manufacturer?
That’s a great question. Usually the company that maintains the technical file and device master record would have the process validation (i.e., sterilization re-validation) records. However, it is possible that you might be outsourcing this to a supplier and they are responsible for it. However, you can’t get CE marking without submitting this data and FDA inspectors would ask for these records. Therefore, even if you outsource the responsibility for the work, you will probably still need to provide copies of the records.
Hi,
I have a question, in-case the re-validation interval (for the EO sterilization process that has only 1 product) was longer than 2 years, which risk aspects we should evaluate for this deviation?
The revalidation interval should be at least every 2 years–not longer. You should evaluate the need for re-validation annually. All aspects that could effect the safety and effectiveness of the sterilization process and your product should be evaluated for potential impact if they have changed. This includes, but it not limited to: PCDs, bioburden, packaging materials, volume of production, load configuration, maintenance, and repairs.