This CAPA training webinar explains how to create a risk-based CAPA process, and you will learn how to implement corrective and preventive actions step-by-step.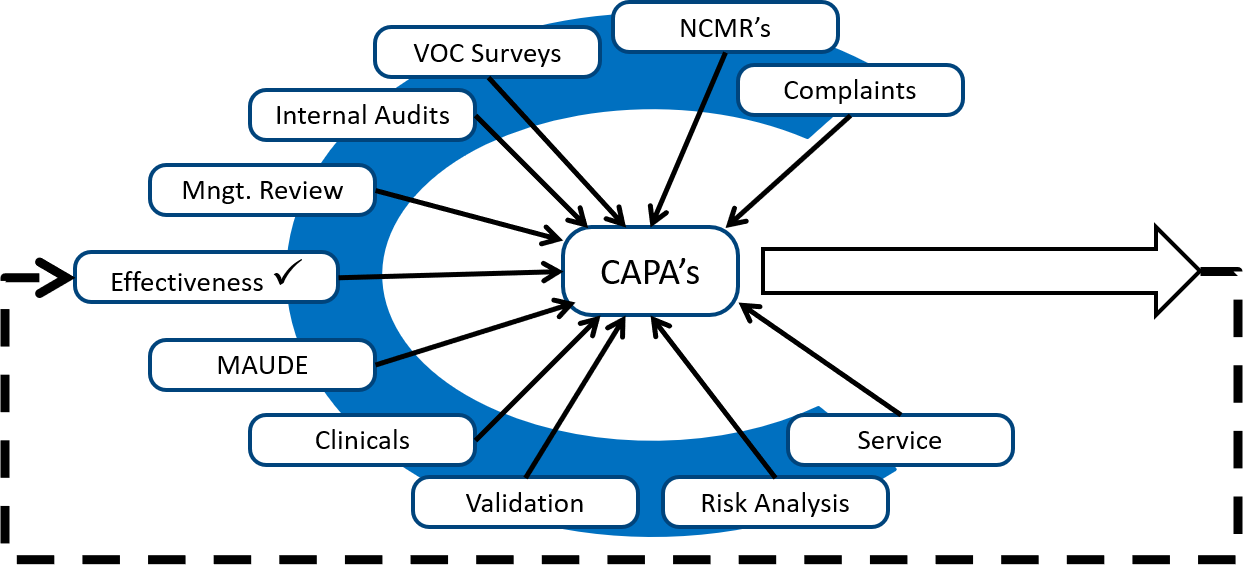
When is CAPA Training?
The CAPA training webinar for risk-based CAPA was recorded on June 30, 2020. Everyone who purchases the webinar will receive a link to download the recording and a copy of the native slide deck.
This CAPA training recording is only $129 (AND INCLUDES NATIVE SLIDE POWERPOINT PRESENTATION FILES):
20- Question Exam and Training Certificate available for $49.00:
Do you also need a CAPA Procedure?
Companies that receive a non-conformity or FDA 483 inspection observation often need a new CAPA procedure as well as CAPA training. Our CAPA procedure, and all of our quality system procedures, are risk-based. Specifically, we use the GHTF grading system to score CAPAs in the same numeric scoring system used for MDSAP audit findings. Therefore, this is the perfect CAPA procedure and form for ISO 13485 certification and MDSAP certification. The CAPA procedure also includes a webinar, but the webinar focus is specific to implementing the procedure and customizing it for your company. In contrast, this risk-based CAPA training webinar explains the details of performing a CAPA investigation, identifying the root cause, writing a corrective action plan, and performing effectiveness checks.
Description of the CAPA training webinar on how to create a risk-based CAPA process
A risk-based CAPA process is a common goal of medical device manufacturers, but until recently “risk-based” was not clearly defined. The two quality management system standards, ISO 9001 and ISO 13485 were revised and re-issued. The current versions are: ISO 9001:2015 released in October 2015 and ISO 13485:2016 released in February 2016. The biggest fundamental change in both standards is an emphasis on risk-based process management. The CAPA process is the heart of your quality system and one of the most important processes. Therefore, this CAPA training gives you a whole new set of tools for managing your CAPA process using a risk-based approach.
A risk-based CAPA process is more than prioritization
This CAPA training goes beyond simple prioritization of CAPAs and color-coding of CAPAs as high, medium, and low risks. Instead, Rob Packard reviews best practices in risk management (i.e., ISO 14971:2019 and ISO/TR 24971:2020) and he applies the more rigorous risk management process to the CAPA process.
This is a “must-see” presentation for anyone that is responsible for the CAPA process or participates in their company’s CAPA Board. Register for our CAPA training and our speaker will help you integrate risk management activities with your CAPA process.
This CAPA training includes:
- Revised requirements for ISO 9001:2015 and ISO 13485:2016
- An outline of the CAPA process and proposed risk management activities
- Various risk control options that can be integrated with corrective actions
- How to reconcile conflicts between the definitions for risk in ISO 9001:2015 and ISO 13485:2016
- Root Cause Analysis Tools
- 5 Why Analysis
- Is/Is Not Analysis
- Fishbone diagrams
- Brainstorming
- Pareto analysis
- Effectiveness checks
- Sources of corrective and preventive actions
- Pareto analysis of service issues
- Trending CAPAs, average aging of CAPAs, “Death by CAPA”
- Risk Management: risk-based CAPAs, risk thresholds, 7 criteria for estimating risk
- Documenting CAPAs
- How to write an effective CAPA SOP
- Auditing the CAPA process
- Problem-solving A3 reports
- Key elements of CAPA forms
VIEW OUR PROCEDURES – CLICK HERE OR IMAGE BELOW:
About Your Instructor
 Rob Packard is a regulatory consultant with 30 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Robert was a senior manager at several medical device companies—including President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009-2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Robert’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510(k) submissions. The most favorite part of his job is training others. Specialties: CE Marking, Canadian Medical Device Applications, Post-Marketing Activities, Supplier Quality, CAPA, Risk Management, Auditing, Sterilization Validation, Lean Manufacturing, Silicone Chemistry, Extrusion, Bioprocess Engineering, and Strategy. He can be reached via phone at 802.258.1881 or by email. You can also follow him on Google+, LinkedIn, YouTube, or Twitter.
Rob Packard is a regulatory consultant with 30 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Robert was a senior manager at several medical device companies—including President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009-2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Robert’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510(k) submissions. The most favorite part of his job is training others. Specialties: CE Marking, Canadian Medical Device Applications, Post-Marketing Activities, Supplier Quality, CAPA, Risk Management, Auditing, Sterilization Validation, Lean Manufacturing, Silicone Chemistry, Extrusion, Bioprocess Engineering, and Strategy. He can be reached via phone at 802.258.1881 or by email. You can also follow him on Google+, LinkedIn, YouTube, or Twitter.



