This Indications for Use webinar helps you select a predicate for your 510k, write your indications for use, and justify any differences.
Content for Indications for Use Webinar
- Where to download the current FDA Form 3881
- Review of Indications Described in Regulations
- Review of Indications Described in Predicate 510k Summary
- More Broad Indications for Use
- More Narrow Indications for Use
Buy the Indications for Use Webinar for only $64.50
As with all of Medical Device Academy’s webinars, we invite you to participate in the live webinar, give you access to the recording to download after the live webinar, and give you a copy of the native slide deck.
When is the FDA eSTAR indications for use webinar?
The updated webinar is being hosted live on August 15, 2024 @ 10:30 a.m. ET. The session will also be recorded. You can purchase it on-demand and watch the training as often as you wish. If you need help preparing your FDA eSTAR submission, we offer a standard fee for 510k submissions or we can help you on an hourly basis.
10-Question Exam & Training Certificate for $49.00:
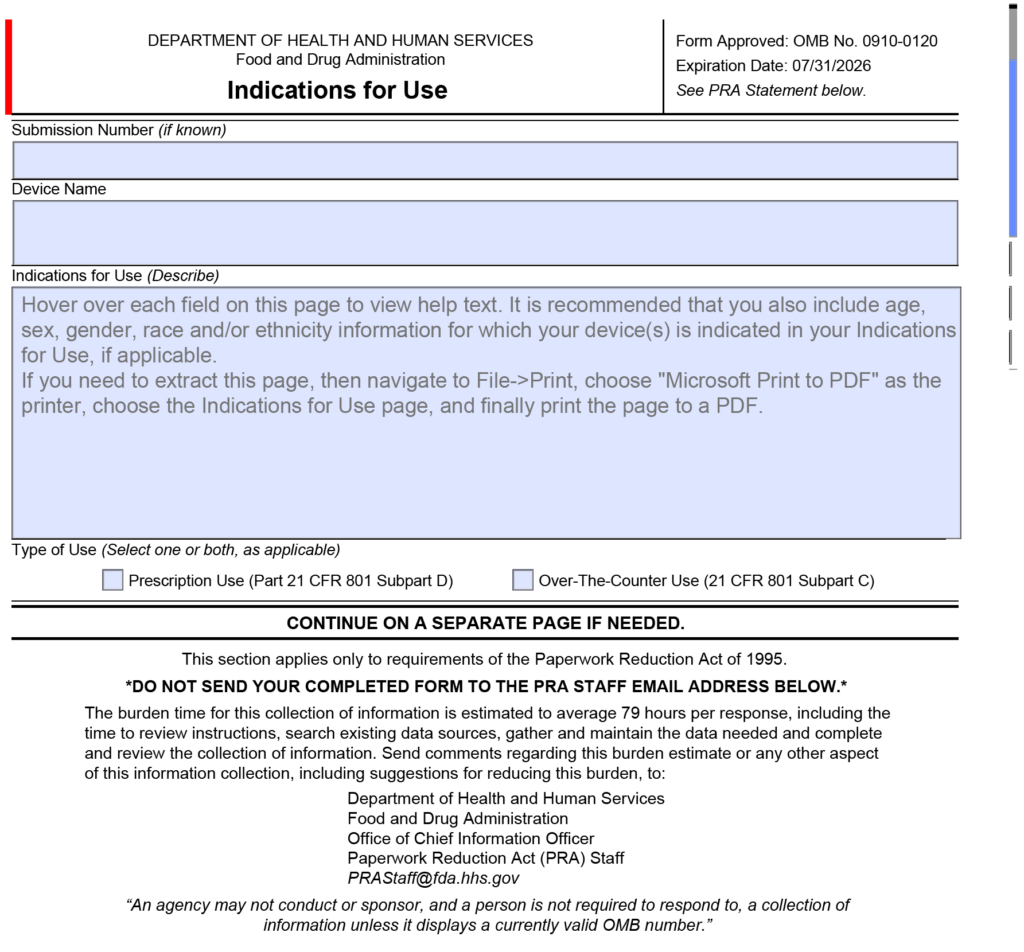
What is FDA Form 3881?
FDA Form 3881 is the form that documents your “Indications for Use” for a medical device submission (i.e., 510k or De Novo). The form is now integrated into the FDA eSTAR and PreSTAR templates (see screenshot below).
What if your Indications for Use are different from the proposed 510k predicate?
If the indications for use for your device are not identical to the predicate device you selected, then you need to justify those differences. That justification will be entered in a text box in the Substantial Equivalence Section of the eSTAR for your 510k submission (see below).

Your justification for differences in the indications for use must also be included in your 510k summary. In the eSTAR, you can automatically generate a 510k summary, which will populate the text in the box above directly into your 510k summary.
510(k) Medical Device Academy Training Overview – Brief Video
Additional 510k Resources Beyond the Indications for Use webinar
If you would like additional training on 510k submissions or you would like to access Medical Device Academy’s templates, you can purchase all of our templates and 510k webinars on our 510k course webpage.
About Your Instructor
Rob Packard is a regulatory consultant with ~25 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Rob was a senior manager at several medical device companies—including the President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009 to 2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Rob’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510k submissions. The most favorite part of his job is training others. He can be reached via phone at +1.802.281.4381 or by email. You can also follow him on YouTube, LinkedIn, or Twitter.



