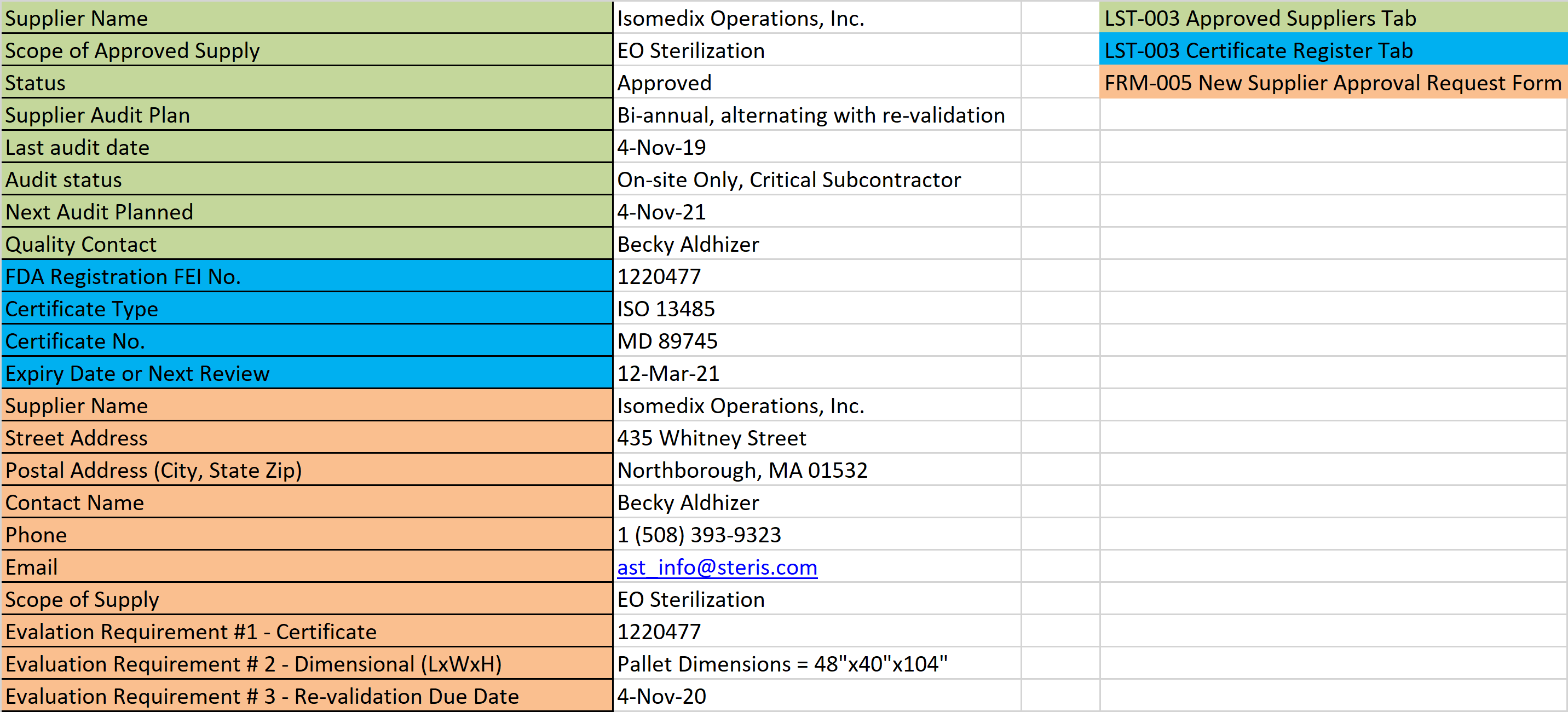
You already have a supplier questionnaire, but do you know how to make a supplier questionnaire to assess a supplier’s ability to support a remote audit?
The four most significant mistakes people make when designing a supplier questionnaire
In Medical Device Academy’s supplier qualification webinar, you learn how to improve your supplier qualification process by replacing the traditional methods of supplier qualification with more effective approaches to supplier evaluation. The following are four examples of how to improve your supplier questionnaire.
Supplier questionnaires should be specific to the product or service provided
The first mistake people make is to use a generic questionnaire. It would be best if you asked your supplier questions that are important to the work that the supplier will be performing. Therefore, each category of product or service should have its own set of questions. For example, important questions related to ethylene oxide contract sterilization services are the maximum size limitations for pallets in the sterilization chamber and whether the facility can conduct sterility testing on-site. However, an injection molding supplier might delay the return of your supplier questionnaire if these questions were on the survey that you send to them because they don’t understand the questions.
Supplier surveys should be more than checkboxes
The second mistake people make is to ask questions that can be answered with a “yes” or “no” response or a checkbox. These are closed-ended questions. It would be best if you always were asking open-ended questions because the response will give you more information about the supplier. In addition, most people resist responding with a “no” response even if the real answer is “no.” For example, “What is your FDA registration number?” is more useful than “Is your company FDA registered?” Another example is, “How many production lines use SPC charts?” instead of “Do you use SPC charts?” In fact, in the open-ended version of this question, you will learn if the use of SPC charts is widespread, and you learn how many production lines the supplier has.
Remember to ask suppliers to update survey surveys every year
The third mistake people make is to request that a supplier questionnaire be completed only during the initial supplier qualification process. Every year companies grow, shrink, or change. If you ask suppliers to update their questionnaire, you can use that information to determine the health of your supplier’s business. You might also discover that one supplier just added a new production capability that will allow you to consolidate more of your outsourced work with that supplier and eliminate another problem supplier. Every company has a turnover in personnel as well. It is a great idea to ask suppliers to provide contact information for multiple people in the organization, such as quality contact, billing contact, and a production planner. Eventually, you will probably need to speak with each of these people, and if one of the contacts is no longer at your supplier, you will still have two other contacts. Updating this information also gives you a hint of whether turnover is widespread or limited to a specific individual.
Supplier questionnaires should be in spreadsheet format
The fourth mistake people make is to send a Word Document for suppliers to complete (PDF format is even worse). Word and PDF formats are time-consuming to complete, and they are harder for you to analyze than a spreadsheet. Most people provide a Word document or a PDF because they are focusing on the requirement for control of records. However, if you have an electronic quality system, the supplier survey information will be part of your electronic system as soon as you enter the data into your software. Alternatively, if you have a paper-based quality system, then you can print the spreadsheet out, sign it, and date it. The huge advantage of using Excel spreadsheets is that you can copy the new data into a column next to the previous year’s responses. Then you can quickly see what changes your supplier made in the past year.
What should you add to your supplier questionnaire?
Most private companies will not share what their revenues are for the business, but as a customer, you should be more concerned with how many human resources your supplier has. Therefore, you should consider asking, “How many employees, or full-time equivalents (FTEs), work for your company?” You might also want to know if your supplier is relying on a temporary workforce. For example, “What percentage of the FTEs are temporary workers?” Many questionnaires will ask for the square footage of the facility, but this doesn’t provide you with any details about the facility layout. Alternatively, you could ask for a copy of the pest-control map for the facility. This would give you a detailed layout of the facility, and it also confirms that your supplier has a pest control plan for the facility. Another related question to ask is, “Please describe any expansion/construction projects that have been implemented in the past year or projects that are in progress (e.g., the addition of a mezzanine).” If the company added 30,000 square feet to their production area, but there was no change to the pest control plan, you might have some clarification questions for your supplier. In general, a good strategy for developing your questionnaire is to think of at least one open-ended question related to each clause of the ISO 13485:2016 standard without referencing the standard. The following are some examples that might help you:
- When was the last software re-validation for quality system software?
- How many active external standards is your company currently maintaining?
- Please provide a list of procedures and identify the person who would be interviewed during an audit for each procedure (i.e., process owner or subject matter expert).
- In the absence of the management representative, who is designated as the liaison for an FDA inspector?
- What are the upper control limits for particulate counts, air viable counts, and surface viable counts in your controlled environment(s)?
- On what dates was the environmental monitoring of controlled environments conducted in the last year?
- Please identify how many quality inspectors are responsible for the incoming inspection?
- Please list the calibration ID and equipment name for any inspection equipment that requires specialized training (e.g., CMM)?
- How many suppliers are on your approved supplier list (ASL)? And how many suppliers did you audit in the past year?
- How many nonconforming material reports (NCMRs) were opened in the past year? And how many NCMRs currently remain open?
- How many partial or complete lots were returned to your company by customers in the past year?
- Please list any corrections and removals (i.e., recalls) that your company has been involved in during the past year and the current status?
How many questions should your supplier questionnaire include?
There are 28 required procedures in ISO 13485:2016, and there are even more subclauses within the standard. It is an excellent idea to create a list of questions you might ask for each subclause, but a supplier questionnaire should not include all of those questions. Just as audits are just a sampling, your supplier survey questions should be sampling as well. You should review last year’s questions and eliminate questions that you think are not especially useful for that supplier. Some questions should be asked each year to assess if the quality system has changed significantly, and you should consider adding a few new questions each year. The best questions will require the person to perform some research to answer the questions. But it is unreasonable to expect a supplier to spend more than two hours completing a supplier questionnaire if you plan to purchase less than $20,000 in product or services.
Supplier questionnaires specific to remote auditing
In many ways, a well-designed supplier questionnaire is similar to a remote audit, because you are asking the supplier to answer multiple open-ended questions about their quality system to verify that the quality system is fully implemented and remains effective. However, due to the Covid-19 pandemic, many employees are now required to work from home, and it is not possible to physically visit certain facilities. Therefore, you should be adding three elements to your supplier questionnaire to assess your supplier’s ability to support a remote audit and to determine their ability to maintain the effectiveness of the quality system during a viral outbreak. The three elements are 1) policies for personal protective equipment for employees and visitors, 2) business continuity plans to maintain internal operations and to ensure redundancy of crucial suppliers, and 3) availability of digital documents and records or paper documents and records via video conference software. These three areas were also the subject of a previous blog on changes triggered by Covid-19. It would help if you also asked about the availability of hardware and software communication tools for conducting a remote audit. You might ask your supplier, “Which areas of your facility can we observe during a remote audit using live video conferencing (e.g., Zoom mobile application)?” and “What experience does your company have in the use of Zoom as a video conferencing tool?”
Access to documents and records during remote audits
During a remote audit, you will need to access documents and records virtually. If your supplier can participate via a video conferencing tool with a high definition web camera or smartphone, then you should be able to see any documents and records that you could normally see during an on-site audit. However, your supplier will need to hold the document or records steady, possibly by using a music stand and a camera tripod so that you can take notes regarding the contents of the document or record. You will also need a way to record your notes. You might try using a Pixelbook or similar computer to write your audit notes. At the same time, you watch the video conference using a second computer–possibly on a conference room projector screen or large flat screen monitor. You could also use a tablet, such as remarkable. Of course, you can always use a pad of paper and a pen and then transcribe your notes later. All of these methods will be faster and more convenient than digitally scanning each document and uploading the documents to a shared folder or sending the scanned document by email.
It would help if you also were asking your supplier which records are already available digitally. You can expect all of the quality system procedures to be available in digital formats, but many records may already be available electronically as well. For example, purchase orders, quality system certificates, drawings, and blank forms should be available in digital format. In a supplier audit, you typically will focus on a subset of the quality system records that are related to production process controls, purchasing, incoming inspection, shipping, and control of the nonconforming product. Asking your supplier which of these records are available in digital format will help you determine which records you need to request from the supplier in advance and which records can be requested on-demand.
How to obtain our supplier questionnaire template (FRM-004)
If you are interested in purchasing our supplier questionnaire template, FRM-004, it is included with the purchase of our supplier qualification webinar. If you think of any new questions to add to this template, please email me at rob@13485cert.com. Just put “FRM-004 Suggestion” in the subject line.