Are you a start-up device company that needs medical device consulting services and training for an FDA 510k submission?
Medical device consulting services
Medical Device Academy, Inc. is a quality and regulatory consulting firm. We have ten (10) employees, and everyone works virtually from home. We specialize in helping small device companies prepare FDA 510k submissions using the eSTAR template, preparing FDA pre-submissions using the PreSTAR template, implementing new quality systems for compliance with the FDA, ISO 13485, and MDSAP, and conducting quality system audits. We also help with FDA US Agent services, CE Marking preparation, and Canadian License applications. Clients with urgent needs where time to market is critical turn to us. Our passion is teaching medical device professionals how to prepare for future regulations. For more information, please visit our website or our YouTube channel. We even wrote a 510k book, “How to prepare your 510k in 100 days,” because 100 days is how long your testing will take. It is only available as part of our 510k course series, and you can find all the details on our 510k course webpage.
Buy the 510(k) course series for $1,495
What do we charge for medical device consulting to prepare an FDA 510k submission?
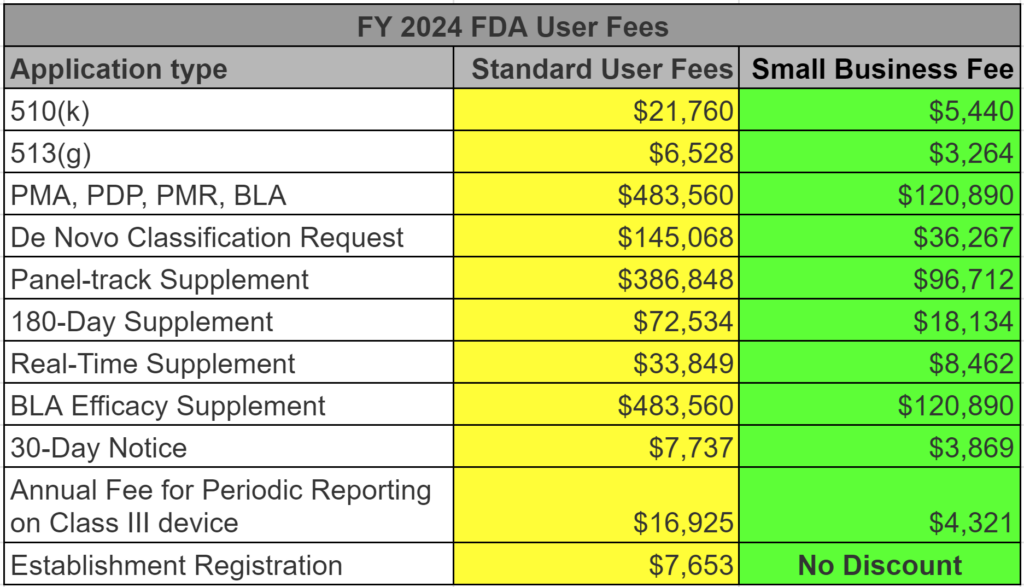
We charge $17,500 for preparing a medical device 510k submission. This pricing does not include pre-submission meetings or the FDA user fees for FY 2024. A table with user fees is found below as well. A button for downloading our standard pricing sheet for all services is provided below.
We are affordable because we know your start-ups have “one egg and one basket.” We are casual. We like taking risks. We are irreverent. We make mistakes, but we learn from them. We want our work to be fascinating. We teach the most boring topic on planet Earth, but we refuse to be boring. We want to be remarkable, memorable, and fun to work with. Our team is primarily located in the Eastern Time Zone, with medical device consultants in North Carolina, Pennsylvania, New Jersey, Kansas, and Vermont. We all work 100% remotely, and we always have.
FDA small business qualification for FDA submissions
The FDA user fees for medical device 510k submissions, 513(g) submissions, and De Novo Classification Requests are all discounted for qualified small businesses. To qualify, you must submit a small business qualification form (FDA Form 3602 or 3602A) each new fiscal year (October 1 – September 30). We recommend that every company submit on August 1 for the next fiscal year–even if they are unsure if they will submit. This advice alone will save your firm thousands of dollars. Below is a table listing all of the FDA user fees for submissions–both standard and small business fees.
Who is our ideal medical device consulting client?
We are a full-service medical device regulatory consulting firm. The name “academy” emphasizes our love for training. We specialize in helping entrepreneurs get their first product to market–regardless of whether that is a medical device 510k clearance or a De Novo Classification Request. Ideally, you are located in another country, and you need help understanding the requirements of the US FDA. The best time for you to contact us is at the early stages when you have a proof-of-concept prototype but are unsure what to do next. You also might be under-capitalized, and you need advice on how much to budget and how to use your limited funding most efficiently.
How long does it take to obtain a medical device 510k clearance?
Overall, the average is 125 days, but you can only estimate the time to obtain medical device 510k clearance by analyzing the data from recent, previous submissions for the same product classification. Every classification is different, and the average for each product classification ranges from less than 90 days to more than 180 days. We can explain the various options for reducing the review time (e.g., third-party reviewers), but the biggest delays are caused by the need to repeat testing because clients skipped a pre-submission meeting.
Medical device product classifications and regulatory pathways
The FDA regulatory pathway and other regulatory pathways are dependent upon the classification of the device. We provide regulatory pathway analysis services to clients for the US market, the European market, and the Canadian market. We also help you determine the testing requirements, identify potential predicates, and explain the quality system requirements for your type of device. This is typically included with our medical device 510k pre-submissions and De Novo Applications.
FDA substantial equivalence (SE) decision process
The process flow diagram in the video above may seem simple, but how you interpret each question can change the answer significantly. Sometimes, a few words or a single design feature can change the FDA reviewer’s decision when evaluating substantial equivalence. Many of these differences concerning interpretation become evident during the pre-submission meeting with the FDA. Following the wrong path will result in significant delays at best, and many clients discover that clinical testing and/or a De Novo Application is required due to changes in technological characteristics or due to a slight change in the indications for use.
Developing a verification and validation (V&V) testing plan
Having the right verification and validation testing plan is critical to timely FDA 510k clearance and approval of De Novo Requests. That’s why we prepare draft testing plans for clients, and then we ask the FDA for feedback on the testing plan during a pre-submission meeting. We use guidance documents, competitor submissions, and our experience with similar devices. However, we often learn something new from the FDA in every pre-submission meeting because the requirements constantly change.
Medical device 510k project management
Most people see meetings as a waste of time, but we try to conduct efficient, weekly, 30-minute meetings with medical device consulting clients. The purpose is to answer their questions, give them an update on the project status, and make sure there are no surprises that could delay the project’s completion. Typically, we are waiting for the final testing reports, such as 1) the sterilization validation report, 2) the sensitization testing report, or 3) the electrical safety testing report. For every project, we use the table of contents as a color-coded planning tool to track the status of every document needed for your submission.
What do our quality and regulatory consultants do?
Most of our work is preparing regulatory submissions, but 20% of our work involves helping you implement your quality system. We can help you with your medical device regulatory submissions. We specialize in medical device 510k submissions, preparation of CE Marking Technical Files, and Canadian device license submissions. We also created a business unit that specializes in preparing and validating FDA eCopies for pre-submissions, 510k submissions, and De Novo Applications. Due to changes in the FDA eCopy policies, we no longer offer FDA eCopy services except to active medical device consulting clients. If you need to upload your own FDA eCopy, you can register for a Customer Collaboration Portal Account with the FDA so that you can upload your own submission documents electronically.
Most of our clients are small and mid-size medical device companies that need continuing education training and quality System Auditing for compliance. Clients with urgent needs where time to market is critical turn to us. Our passion is teaching medical device professionals how to prepare for future regulations and self-monitor compliance to avoid compliance remediation.
How are we different from other quality and regulatory consultants?
Most medical device consultants work by the hour, while our firm has flat-fee pricing. We can do this because we specialize in a narrow niche–primarily medical device 510k submissions and De Novo requests. We also differ from independent consultants and law firms because we have designed and built medical devices. Rob Packard was CEO and co-founder of a medical device start-up in 2004.
Contacting our medical device consulting team
Typically, medical device consulting clients schedule calls using our calendly app. Rob typically starts his day with calls to European clients and Western Asia around 6 am and calls with the West Coast often extend past 6 pm. If you need a time that is earlier or later, please just send us an email. Our Skype and Google Voice numbers are provided in the above video as well. If you are looking for testimonials, please visit our testimonials page.


