This human factors training webinar will teach you how to perform a task analysis to identify critical tasks for your medical device 510(k).
Task Analysis Template & Webinar Bundle – $129
When is the Task Analysis webinar scheduled?
This webinar will be live on Thursday, October 17, 2024 @ Noon ET. The session will also be recorded. You can purchase it on-demand and watch the training as often as you wish.
Why should you consider registering for this Task Analysis Webinar?
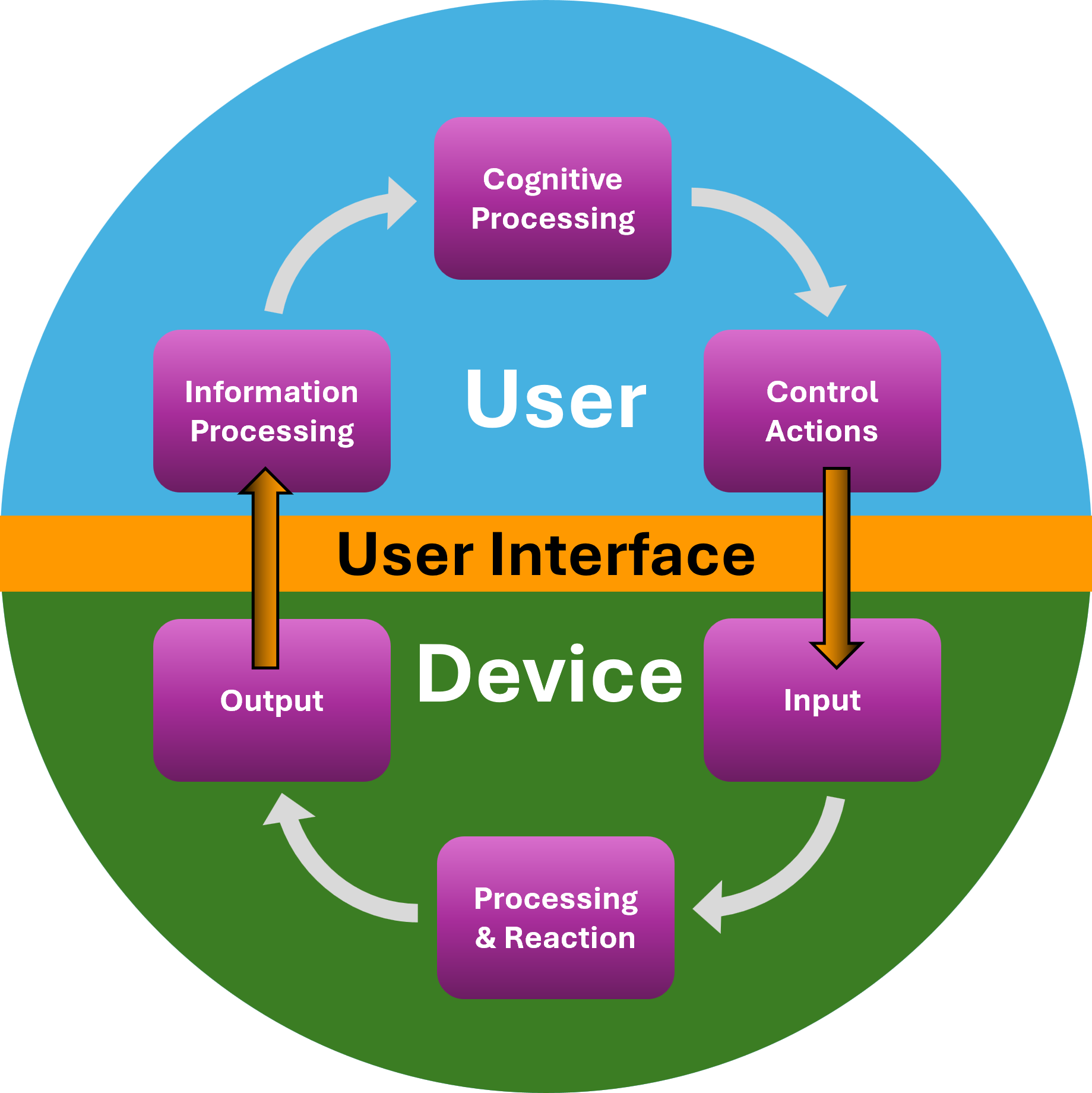
One of the steps in creating a usability engineering file is to perform a task analysis. Matthew Walker and Rob Packard will explain the overall process for creating a usability engineering file and how the task analysis fits into the human factors process and how it’s included in your 510k submission using the FDA eSTAR template. They will show you how to complete Medical Device Academy’s task analysis template, walk you through the new work instruction for this template, and present a few cases studies to help you understand the process. Live attendees will also be able to ask questions.
What you will receive for $129
- Task Analysis Template
- Login information for the live Task Analysis Webinar
- Work instruction for performing a task analysis
- Access to download the recording of the webinar from our Dropbox folder
- Native slide deck for this webinar
All content deliveries will be sent via AWeber emails to confirmed subscribers.
Other Usability Engineering / Human Factors Training
- Human Factors Training Series – $950
- Formative Usability Testing Webinar & Template Bundle – $79
- Use Error and Abnormal Use Decision Tree Training – $79
- Use Specification Template & Webinar Bundle – $79
- Use-Related Risk Analysis (URRA) Template & Webinar Bundle – $299
- Participant Screening & Data Collection Forms – $79
- Summative Usability Testing Protocol & Webinar Bundle – $199
- Summative Usability Testing Report – Live-streaming Free Webinar
About Your Instructors
 Matthew Walker – QMS, Risk Management, Usability | Human Factors Engineering, Cybersecurity & DFIR
Matthew Walker – QMS, Risk Management, Usability | Human Factors Engineering, Cybersecurity & DFIR
Matthew brings a unique background as a former Firefighter/EMT and Rope Rescue Tech with experience in OSHA and NFPA regulations. For the better part of a decade, he has worked as a Technical/Medical Writer and Lead Auditor. He holds degrees in Fire Science and Computer Forensics and Digital Investigations, graduating Summa Cum Laude from Champlain College. Matthew is also an active member of several academic honor societies including Omicron Sigma Sigma’s Order of the Sword and Shield. His professional focus includes Human Factors Engineering, Risk Management, and Cybersecurity with a special interest in applying Digital Forensics and Incident Response (DFIR) practices to medical technology. He combines regulatory expertise with technical insige to strengthen both product safety and oranizational resiliance. He can be reached by email. You can also follow him on LinkedIn or YouTube.

Rob Packard is a regulatory consultant with ~25 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Rob was a senior manager at several medical device companies—including the President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009 to 2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Rob’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510k submissions. The most favorite part of his job is training others. He can be reached via phone at +1.802.258.1881 or by email. You can also follow him on YouTube, LinkedIn, or Instagram.