This page includes the information you need for small business qualification (a.k.a., SBD) to obtain a 75% FDA User Fee discount.

Many small businesses don’t yet earn revenues, but if you plan to submit a 510k, you might want to file your taxes–even if you don’t need to. The new FDA user fees have been released, and if your small business has a tax return, you can qualify for a 75% discount if you submit your small business qualification application. As of October 2024 your small business qualification documents must be uploaded to the FDA as an eSubmission through the FDA CCP to obtain a small business determination (SBD).

Note: The above dollar amounts are effective from October 1, 2025, to September 30, 2026. The full list of FDA User Fees for FY 2026 are provided on our homepage.
Application Form for Small Business Qualification
The small business qualification must be renewed each year. Most small businesses we work with fail to submit the form early enough to take advantage of this deduction, or the companies have difficulty gathering the tax records required for the application. You can download the applicable forms and guidance from our website using the links provided below (Updated July 2025):
- Link to FDA Form 3602N for US Companies, Subsidiaries, Parent Companies, and Foreign Companies (New July 2025 Universal Form)
- Link to FDA July 2025 Guidance
You will need your organization ID # to complete the application form. Below is a YouTube video that explains how to obtain your organizational ID #. If your firm is outside the USA, you will also need a DUNS #.
How do you submit the small business application?
As of October 2024 your small business qualification documents must be uploaded to the FDA as an eSubmission through the FDA CCP to obtain a small business determination (SBD). Before FY 2025, all small business qualification applications had to be submitted as an original hardcopy to the FDA. If you are unable to use the CDRH portal, the physical address is provided below:
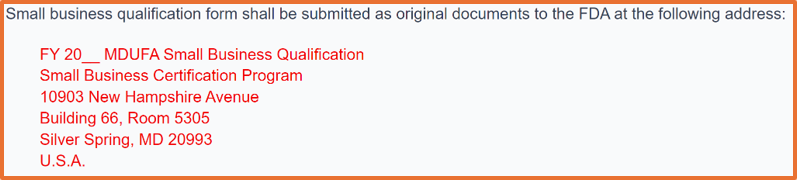
FY 2026 begins on October 1, 2025. If you plan to submit a 510k after September 30, 2026, you must wait until August 1, 2026, because that is the earliest you can apply for FY 2027 small business determination (SBD). The FDA target is to process your request within 60 calendar days, but the turnaround time has been improving at the FDA.
Payment Methods for the FDA User Fees
Once you receive the small business qualification (i.e., the SBD identification number) via email, you must enter your User Fee Account (i.e., DFUF) to create FDA Form 3601 to include it in Section 1 of the 510k submission. Form FDA 3601 is also submitted along with the physical payment of the user fee, or the company must pay the fee electronically.
Does small business qualification apply to the Establishment Registration User Fee?
The above question is very popular, but the answer is no. Regardless of the size of your company or the number of products you distribute, there is only one fee for each establishment to be registered with the FDA. If you would like assistance with registering and listing your company, you can schedule an appointment with Medical Device Academy to help you with this. We have a blog post to help you if you want to do it yourself. We also offer US Agent services for companies located outside the USA.
Additional 510k Resources
If you would like additional training on 510k submissions, or you would like to access Medical Device Academy’s templates, you can purchase all of our templates and 510k webinars on our 510k course webpage.
About the Author

Rob Packard is a regulatory consultant with ~25 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Rob was a senior manager at several medical device companies—including the President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009 to 2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Rob’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510(k) submissions. The most favorite part of his job is training others. He can be reached via phone at +1.802.258.1881 or by email. You can also follow him on YouTube, LinkedIn, or Twitter.

