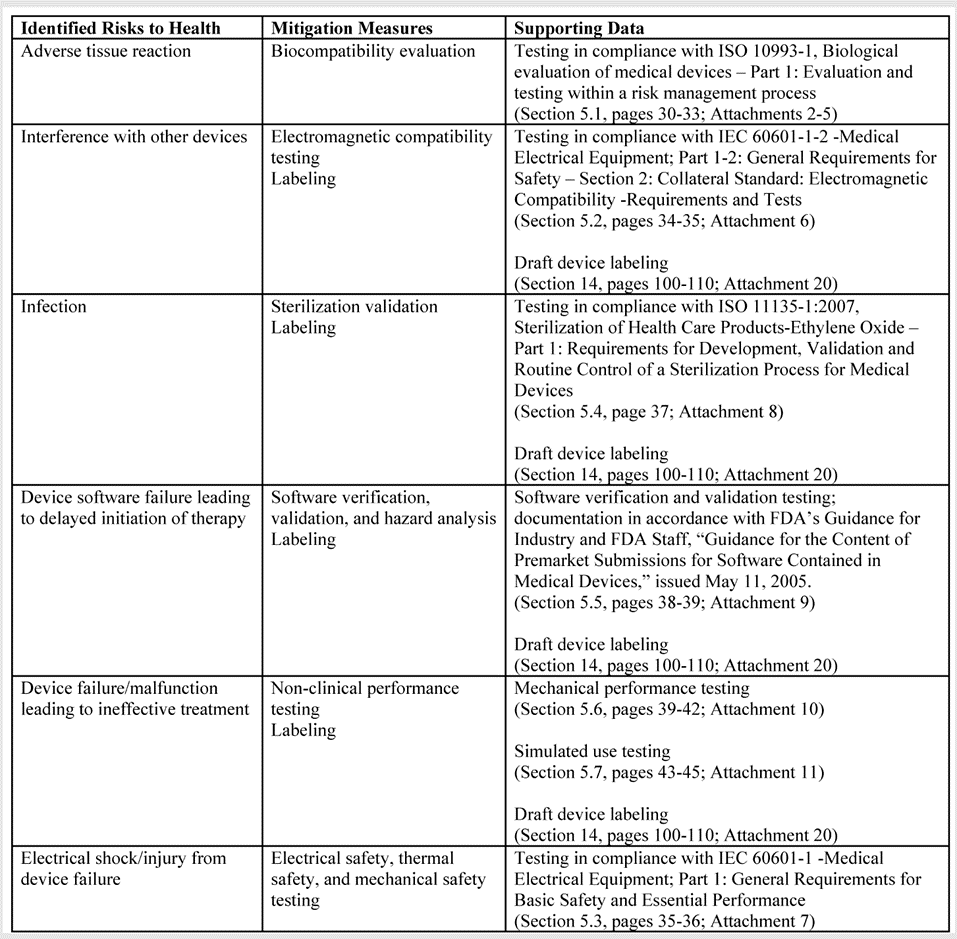
This webinar teaches you how to create a Risk Mitigation Table systematically for your FDA De Novo submission.
Creating a risk mitigation table for a De Novo submission Webinar
When is the risk mitigation table webinar?
This webinar will be live on Thursday, June 27, 2024 @ 10:30 am ET. The session will also be recorded. You can purchase it on-demand and watch the training as often as you wish.
What you will receive:
The webinar is included in our 510(k) course. The webinar includes:
- risk mitigation table template (with prefilled examples)
- multiple examples from other De Novo submissions
- native slide deck
- link to download the recording
- login information (if purchased before June 27, 2024)
If you would like to ask specific questions about preparing your test plan, please submit them via email or schedule a call using the calendar app on our contact us page. All deliveries of content will be sent via AWeber emails to confirmed subscribers. If you don’t receive the content automatically, please check your spam folder.
Q&A
Please submit questions to me by email at rob@fdaestar.com regarding the Test Plan Webinar. If you have company-specific questions, please send me a request to set up a private call to discuss your specific issues.
Additional Resources for 510k submissions
If you would like to upgrade your purchase to our entire 510(k) Course, which includes templates and webinars for pre-submissions, 513(g) Classification Requests, 510(k) submissions, and De Novo applications, please contact Lindsey Walker and she will provide you with a quote that includes a credit for your purchase of this webinar.
About Your Instructor
Rob Packard is a regulatory consultant with ~25 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Rob was a senior manager at several medical device companies—including the President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009 to 2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Rob’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510(k) submissions. The most favorite part of his job is training others. He can be reached via phone at +1.802.281.4381 or by email. You can also follow him on YouTube, LinkedIn, or Twitter.