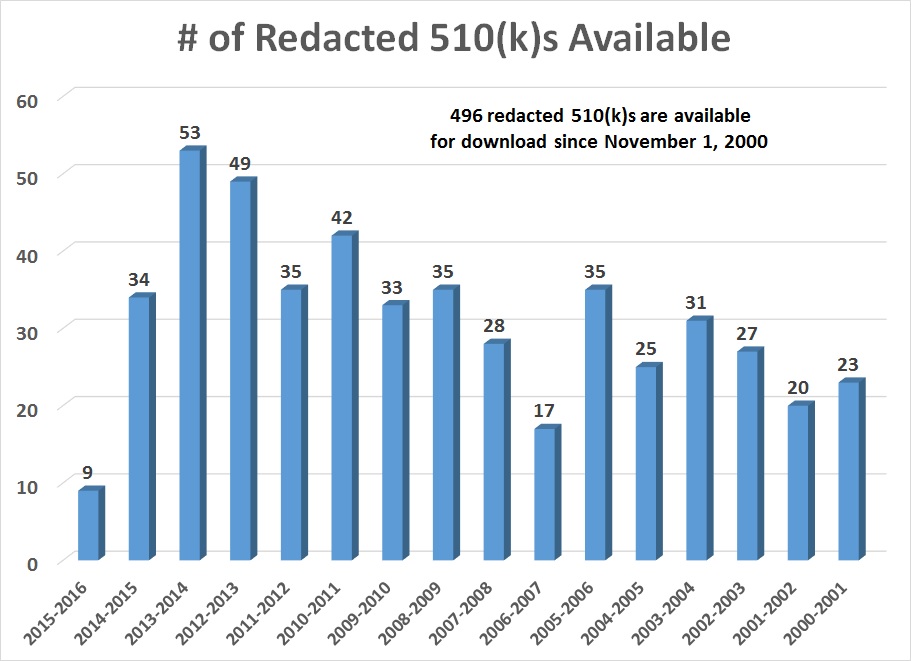
This article describes the new database of redacted 510k submissions recently made available online for immediate download by the US FDA.
Recently, the FDA redacted 510k submissions that were previously released through Freedom of Information Act (FOIA) requests available online for immediate download. 496 redacted 510k submissions have been available since November 2000–as indicated by the graph above. This is only a tiny fraction of the total number of 510k submissions, but the number that is available online will increase over time.
Types of redacted 510k Submissions
Of the 496 submissions, there is a mixture of submission types.
- 382 are traditional 510k submissions
- 97 are special 510k submissions
- 17 are abbreviated 510k submissions
- 14 were 3rd Party reviewed
What remains in a redacted 510k submission
The redacted versions do not include testing data, but you will find other goodies such as:
- 3rd Party SE memorandums (where applicable)
- Table of Contents
- Pre-market Notification Cover Sheet (i.e., FDA Form 3514)
- 510k Cover Letter
- Indications for Use (i.e., FDA Form 3881)
- 510(k) Summary
- Truthful & Accuracy Statement
- Device Description
- Executive Summary
- Substantial Equivalence Discussion (Partially Redacted)
- Summary of Biocompatibility Testing (Partially Redacted)
- Summary of Sterilization & Shelf-Life (Partially Redacted)
- Proposed Labeling
- Predicate Device Labeling
- Declarations of Conformity (i.e., FDA Form 3654)
- Deficiency Letter
This information can be used to help select a potential predicate and develop a verification and validation testing plan. If you are less experienced in preparing a 510k submission, it will help to see how other regulatory experts have organized their 510k submissions.
Learning more about redacted 510k submissions
To access this database, click this link: Redacted FOIA 510k Database. To limit your search to only 510k submissions that are available as a redacted full 510k, click on the box for “Redacted FOIA 510k.” If you want to learn more about how to make the most of this new resource, please sign up for my latest webinar on Monday, November 21 @ 9 am EST.