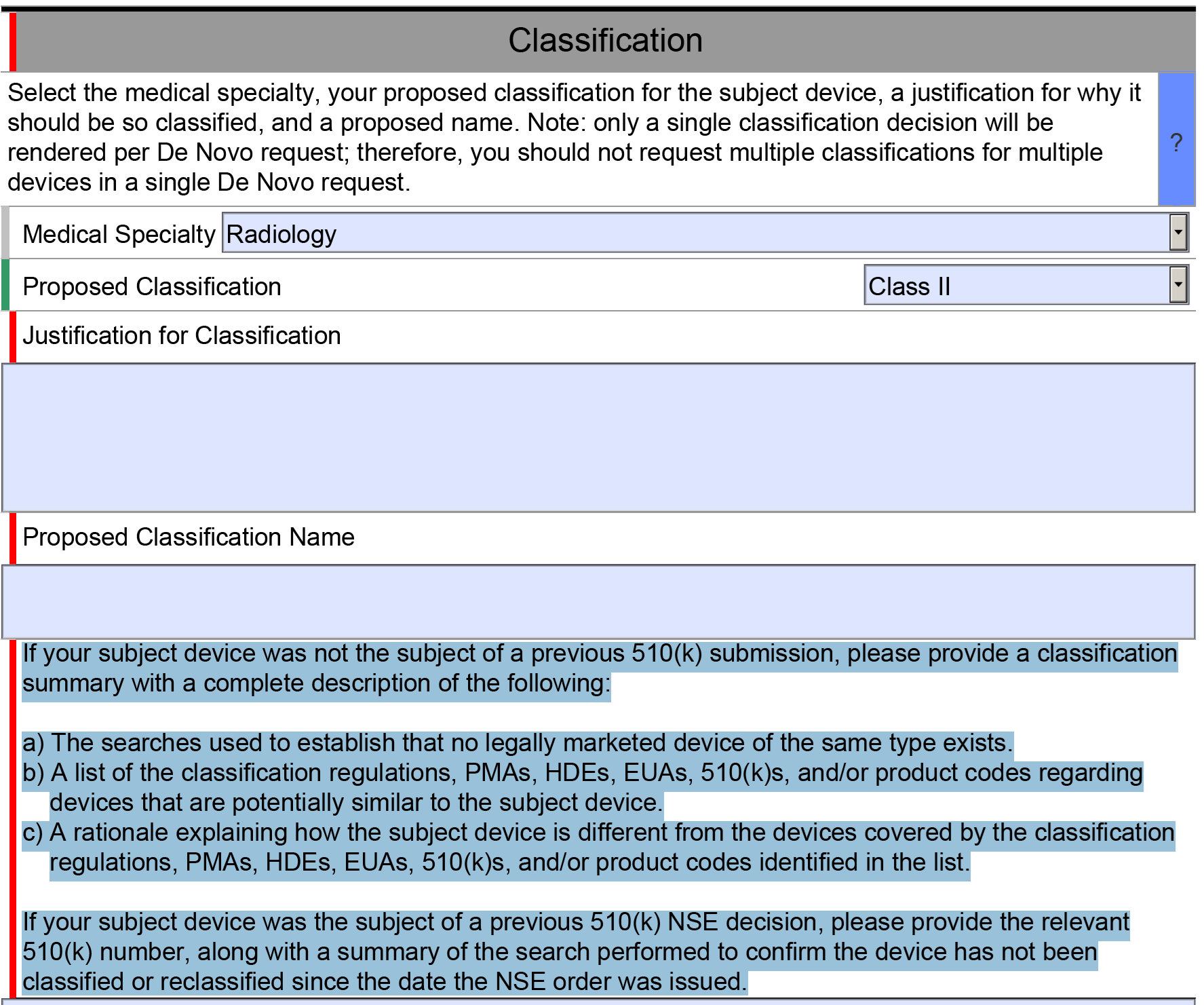
This webinar will teach you how to identify a potential predicate with a template that walks you step by step exactly through what we do.
Identifying a Potential Predicate Webinar ($79)
This webinar will teach you how to identify a potential predicate. We’ve done this before, but now we have a template that walks you step by step exactly through what we do. It shows you screen captures and gives you examples of exactly what we use in the FDA database and other proprietary databases to identify a predicate. You can use this for 510(k) submissions, and for De Novo submissions.
Please note: These products will be delivered to the email address provided in the shopping cart transaction. After the transaction is verified, please check your email for the download.
When is the Identifying a Potential Predicate Webinar?
This webinar will be live on Thursday, July 18, 2024 @ 10:30 am ET. The session will also be recorded. You can purchase it on-demand and watch the training as often as you wish. If you need help preparing your FDA eSTAR submission, we offer a standard fee for De Novo submissions or we can help you on an hourly basis.
What you will receive in the Identifying a Potential Predicate Webinar:
- Efforts to Identify a Potential Predicate Device Template
- Native Slide Deck
- Recording of the Webinar (download and watch again and again)
- Login credentials for the live webinar if you purchase on or before July 18, 2024
If you would like to upgrade your purchase to our entire 510(k) Course, which includes templates and webinars for pre-submissions, 513(g) Classification Requests, 510(k) submissions, and De Novo applications, please contact Lindsey Walker and she will provide you with a quote that includes a credit for your purchase of this webinar.
About Your Instructor
Rob Packard is a regulatory consultant with ~25 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Rob was a senior manager at several medical device companies—including the President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009 to 2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Rob’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510(k) submissions. The most favorite part of his job is training others. He can be reached via phone at +1.802.281.4381 or by email. You can also follow him on YouTube, LinkedIn, or Twitter.