Process monitoring is required but do you know whether monitoring every procedure is required by the FDA QSR or ISO 13485?
One of the elements that Medical Device Academy has incorporated into each procedure we created in our turnkey quality system is a section titled, “monitoring and measurement.” The purpose of this section is to force each process owner to identify a process metric for monitoring every procedure. In some cases, we suggest a metric that would be appropriate for most companies establishing a new quality system. In other procedures, we use the following default text:
“Enter a quality metric that you want to track for this process in accordance with ISO 13485:2016, Clause 8.2.5 and the procedure for Monitoring, Measurement, and Analysis (SYS-017).”
Where are the requirements for process monitoring in 21 CFR 820?
Some of the companies that have purchased our turnkey quality system have asked, “Is it required to monitor and measure something in every procedure?” In general, it is not a specific requirement to have a metric specified in each procedure. In fact, if your quality system is not ISO 13485 certified, there are actually only a few places where the US FDA requires monitoring. The FDA does not have a section specific to monitoring and measurement of processes, but there is a section of the regulations specific to statistical techniques (i.e. 21 CFR 820.250). However, it does not state in the QSR that statistical analysis is required for all processes. In fact, there are only six instances where the word “statistical” is used:
- 21 CFR 820.100(a)(1) – “Analyzing processes, work operations, concessions, quality audit reports, quality records, service records, complaints, returned product, and other sources of quality data to identify existing and potential causes of nonconforming product, or other quality problems. Appropriate statistical methodology shall be employed where necessary to detect recurring quality problems;”
- 21 CFR 820.200(b) – “Each manufacturer shall analyze service reports with appropriate statistical methodology in accordance with § 820.100.”
- 21 CFR 820.250 – “(a) Where appropriate, each manufacturer shall establish and maintain procedures for identifying valid statistical techniques required for establishing, controlling, and verifying the acceptability of process capability and product characteristics. (b) Sampling plans, when used, shall be written and based on a valid statistical rationale. Each manufacturer shall establish and maintain procedures to ensure that sampling methods are adequate for their intended use and to ensure that when changes occur the sampling plans are reviewed. These activities shall be documented.” Note: the other two instances are the title of 21 CFR 820.250.
The word “monitoring” is equally rare (i.e. 4x) in the QSR:
- 21 CFR 820.70(a) – “Each manufacturer shall develop, conduct, control, and monitor production processes to ensure that a device conforms to its specifications…Where process controls are needed…(2) Monitoring and control of process parameters and component and device characteristics during production.”
- 21 CFR 820.75(b) – “Each manufacturer shall establish and maintain procedures for monitoring and control of process parameters for validated processes to ensure that the specified requirements continue to be met…(2) For validated processes, the monitoring and control methods and data, the date performed, and, where appropriate, the individual(s) performing the process or the major equipment used shall be documented.”
Where are the requirements for process monitoring in ISO 13485:2016?
ISO 13485:2016 has a section specific to monitoring and measurement of processes (i.e. Clause 8.2.5). In addition, the word “monitoring” occurs 52 times in the standard and there are 60 incidents of some variant or the exact word. , but there is a section of the regulations specific to statistical techniques (i.e. 21 CFR 820.250). However, it does not state in the QSR that statistical analysis is required for all processes. In fact, there are only six instances where the word “statistical” is used. There are four Clause headings that actually include the word monitoring:
- Clause 7.6, Control of monitoring and measuring equipment
- Clause 8.2, Monitoring and measurement
- Clause 8.2.5, Monitoring and measurement of processes
- Clause 8.2.6, Monitoring and measurement of product
In Clause 1, Scope, and Clause 4.1.5, the Standard states that any outsourced processes remain the responsibility of the company and must be accounted for in the quality system by monitoring, maintaining, and controlling the processes.
Monitoring of risk is included in the definition of “risk management” in the Standard (i.e. Clause 3.18).
Clause 4.1.3 states that the organization shall, “b) ensure the availability of resources and information necessary to support the operation and monitoring of these processes…d) monitor, measure as appropriate, and analyze these processes.”
Clause 4.2.3 states that the contents of the Medical Device File (i.e. MDR or TF), shall include, “d) procedures for measuring and monitoring.”
Monitoring and measurement of processes and product are required inputs to the Management Review in Clauses 5.6.2e) and f).
Clause 6.4.1 requires a procedure for monitoring the work environment if it can have an effect on product quality.
Clause 7.1 requires the company to consider including monitoring in product realization planning.
Clause 7.4.1 requires a plan for monitoring suppliers.
Clause 7.5.1 requires monitoring production and service, including the monitoring of process parameters and product characteristics.
Clause 7.5.6 requires monitoring of validated process parameters.
Clause 7.5.8 requires identification of status with regard to product monitoring and measurement (i.e. inspection status).
Clause 7.6 requires monitoring and measurement of calibrated devices and validation of any computer software used to monitor calibrated devices.
Clause 8.1 states that companies shall plan and implement monitoring and measurement of processes.
Clause 8.2 is titled, “Monitoring and measurement.”
Clause 8.2.1 requires monitoring of customer feedback.
Clause 8.2.5 requires monitoring of processes to ensure planned results are achieved.
Clause 8.2.6 requires monitoring of products to ensure product requirements have been met.
Clause 8.4 requires data analysis of monitoring data from at least six different processes:
- Feedback
- Conformity to product requirements
- Characteristics and trends of processes and products, including opportunities for improvement
- Suppliers
- Audits
- Service reports, as appropriate
In summary, while not every single clause that requires a procedure includes a requirement for monitoring, there are a number of processes where the requirement to monitor the process is explicitly stated.
Why do all of our procedures include the requirement for metrics?
Medical Device Academy expanded the requirement for monitoring to all procedures for five reasons:
- Quality objectives must be “established at relevant functions and levels within the organization.” Therefore, establishing monitoring requirements for each procedure ensures that top management has metrics for every process and a lack of data is never an excuse for not establishing a new quality objective when improvement is needed.
- If every procedure has a requirement for monitoring, then employees don’t have to remember which processes require monitoring and which processes do not explicitly require monitoring.
- The process approach to auditing includes metrics of the process as one of the seven items that are included in every process turtle diagram, and therefore, including metrics for each procedure facilitates the process approach to auditing.
- If a company does not have a process metric already established, it is often difficult to perform an investigation of the root cause of quality issues. If a metric is already being monitored for the process, this facilitates the investigation of the root cause and you can use the baseline monitoring data to help you establish effectiveness criteria for the corrective action.
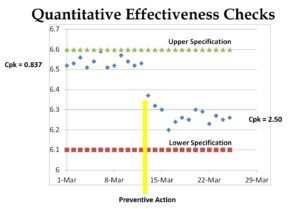
- Finally, most companies struggle to identify preventive actions as required by Clause 8.5.3, and we have found that data analysis of monitoring data is the best source of identifying new preventive actions.
What are the disadvantages when you monitor and measure something in every procedure?
The primary reason for resistance to identifying a metric for monitoring in every procedure is that it will increase the workload for the employees responsible for that process. However, monitoring of data does not always increase workload. In fact, when process data is recorded in real-time on a run chart it is often possible to identify a trend much earlier than when data is simply recorded and subjected to monitoring.
- Example #1: The automatic tracking of toner in a printer tells HP when to ship you a new toner cartridge before you need it. This ensures that there is no loss in productivity because you never run out of ink or the ability to print documents.
- Example #2: Companies will use project management software (e.g. Monday.com) to monitor labor utilization. This will help identify when a specific resource is nearing capacity. When this occurs, the project manager can add time buffers to prerequisite steps and adjust the starting date of the resource-limited tasks to an earlier starting date. This ensures that more time is available to finish the task or to take advantage of resource availability at an earlier date.
- Example #3: Monitoring the revision date for procedures helps the document control process owner identify procedures that should be evaluated for the need to be revised and updated. Often this is articulated as a quality objective of reviewing and updating all procedures within 2 years. This also ensures that procedures remain current and compliant with regulatory requirements.
What are the advantages of monitoring every procedure?
The phrase “what gets measured gets managed” is a popular business philosophy that implies measuring employee activity increases the likelihood that employees will complete a task or perform it well. In contrast, if a process is not monitored, employees may assume that it is not important and the tasks may be skipped or completely forgotten. Setting quantitative goals is also sometimes integrated with economic incentives or bonuses that are granted to individuals and teams.
FDA transition from QSR to ISO 13485
The US FDA is planning its transition from 21 CFR 820 to ISO 13485 as the quality system criteria. This will force companies to make adjustments to their quality systems and increase the amount of process monitoring performed. My general advice is to work with employees that are performing tasks to identify streamlined methods for monitoring those tasks without being overly burdensome. Then you and the employees you manage can analyze the data together and identify opportunities for improvement. When you do this, experiment with manual methods using whiteboards and paper charts that are visible in public areas first. Only implement automated solutions after you have optimized the data being collected and the frequency of data collection, and remember that not every process will benefit from automated statistical process control. Sometimes the simple approach is best.


Pingback: Nine easy ways to organize and improve quality system procedures Medical Device Academy