The complaint handling procedure and webinar will teach you how to process complaints and document them for regulatory reporting.
When is the Complaint Handling Webinar?
The updated complaint handling procedure and webinar are now only available as a bundled digital product. The updated webinar was hosted live on January 4, 2023. A link to download a recording of the live training will be delivered by email after the live event to train other people in your company.
Procedure & Webinar Bundle – Buy Now for $299
Please note: This product will be delivered to the email address provided in the shopping cart transaction. After the transaction is verified, please check your email for the download.
Complaint handling is one of the most common FDA 483 inspection observations. Therefore, we created this procedure and webinar bundle to help your complaint handling unit comply with 21 CFR 820.198 and ISO 13485:2016, Clause 8.2.2. This webinar was originally recorded as a free live webinar on October 4, 2015. The following items are included in this webinar bundle:
- SYS-018 v0.3, Customer Feedback and Complaint Handling Procedure (Procedure in Word format)
- LST-011 v0.1, Complaint Register (i.e., Complaint Log in Excel format)
- FRM-020 v0.3, Complaint Record (Protected Form in Word format – no password)
- a native slide deck for the webinar
- a link to download the recording of the webinar
- a quiz and training certificate
The procedure does not include regulatory reporting requirements that are found in our other related procedures:
- SYS-029, Medical Device Reporting (MDR) Procedure (USA)
- SYS-036, Vigilance Reporting Procedure (EU)
- SYS-035, Incident Reporting Procedure (Canada)
- WI-003, Electronic Submissions Gateway (ESG) Work Instruction
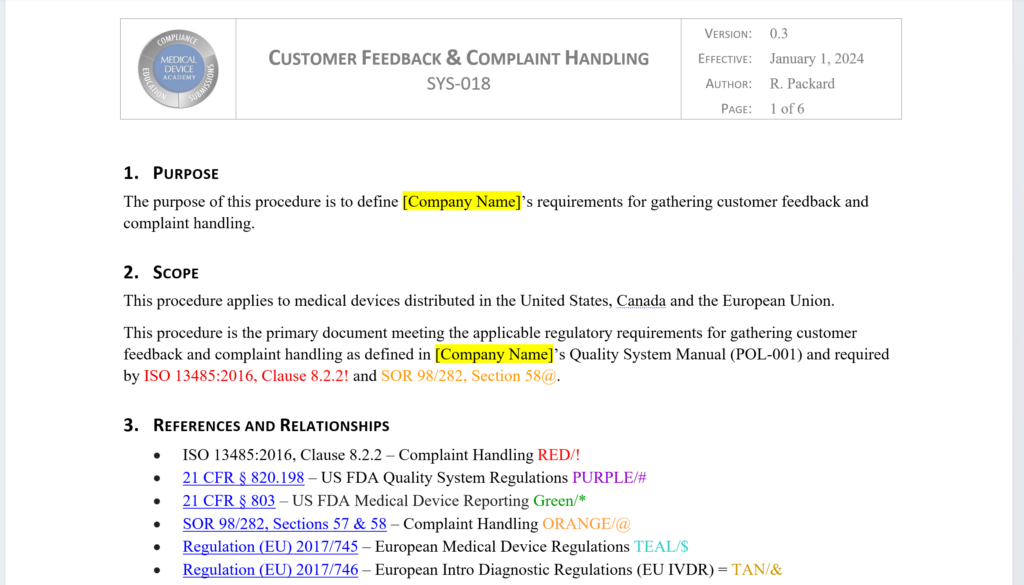
This procedure is the primary document meeting the applicable regulatory requirements for gathering customer feedback and complaint handling as defined in your company’s Quality System Manual (POL-001).The revised and updated procedure is now six pages. The procedure define your company’s requirements for gathering customer feedback, documenting product complaints, and performing complaint investigations. The procedure is updated to include the requirements for the US FDA (21 CFR 820.198), ISO 13485:2016, Health Canada (SOR 98/282, Sections 57 & 58), and both of the European Regulations. Cross-references to each of these requirements was added to the procedure to demonstrate compliance and facilitate certification audits. Each reference is color coded and uses symbols that can be digitally searched (i.e., [CRTL+F]). The complaint record (i.e., FRM-020) was also completely re-written (now seven pages). The updated form now includes 100% of the requirements in the US FDA’s MedWatch Form 3500A. This ensures that complaint records include all the information that is needed for regulatory reporting if you determine that reporting is required.
To view all available procedures click here
To review a sample Medical Device Academy procedure click below:


