FDA US Agent – What do they do?
Medical device companies exporting devices into the USA must have a US agent to register, but what does an FDA US agent do?
What does an FDA US agent do?
Every medical device company outside the USA that distributes devices in the USA must have an FDA US agent. This includes manufacturers, contract manufacturers, and specifications developers outside the USA. The US agent assists the FDA in communication with the device company. The most common communications concern questions about devices exported to the US and scheduling FDA inspections. The role of the US agent is very similar to a European Authorized Representative, a UK Responsible Person, or a Swiss Authorised Representative. Unlike an EC Representative, you do not include US agents in your device labeling. The US agent’s name and contact information only appear on your FDA Establishment Registration record on the FDA website.
Is there any certification or contract required for a US agent?
FDA US agents have no certification process, but you should have a formal signed agreement or contract with your agent. I have never seen the FDA request a copy of the contract or a letter from a US agent or the company that is registered. However, since the agent has a legal role and responsibility, you should ensure an agreement or contract is in place. The agreement or contract should include the following elements:
- Scope of service
- Commitment to perform US agent services promptly
- Duration of service (i.e., specific start and end dates)
- Termination provisions
- Consulting Fees for US agent services (typically an annual fee ranging from $250-$1,500)
- Any additional consulting fees if the FDA contacts your agent
- Who is responsible for payment of FDA User Fees ($11,423 for FY 2026 FDA User Fee)
- Commitment to communicating complaints, especially for potential risks to public health, serious injuries, or death, directly to your company
- Confidentiality clause or reference to a separate confidentiality agreement (Note: The agent may be compelled to disclose information they have to the FDA, but they should notify your company first if this happens.)
- Non-solicitation of your customers or suppliers and no solicitation of employees
- Force Majeure clause
- Identification of the agent’s name, address, phone, and email
- Identification of the company name, address, phone, DUNs Number
- Identification of the company contact’s name, title, address, phone, and email
- Identification of who will be the “Official Correspondent” in the FDA Registration Database
- Signature and Date
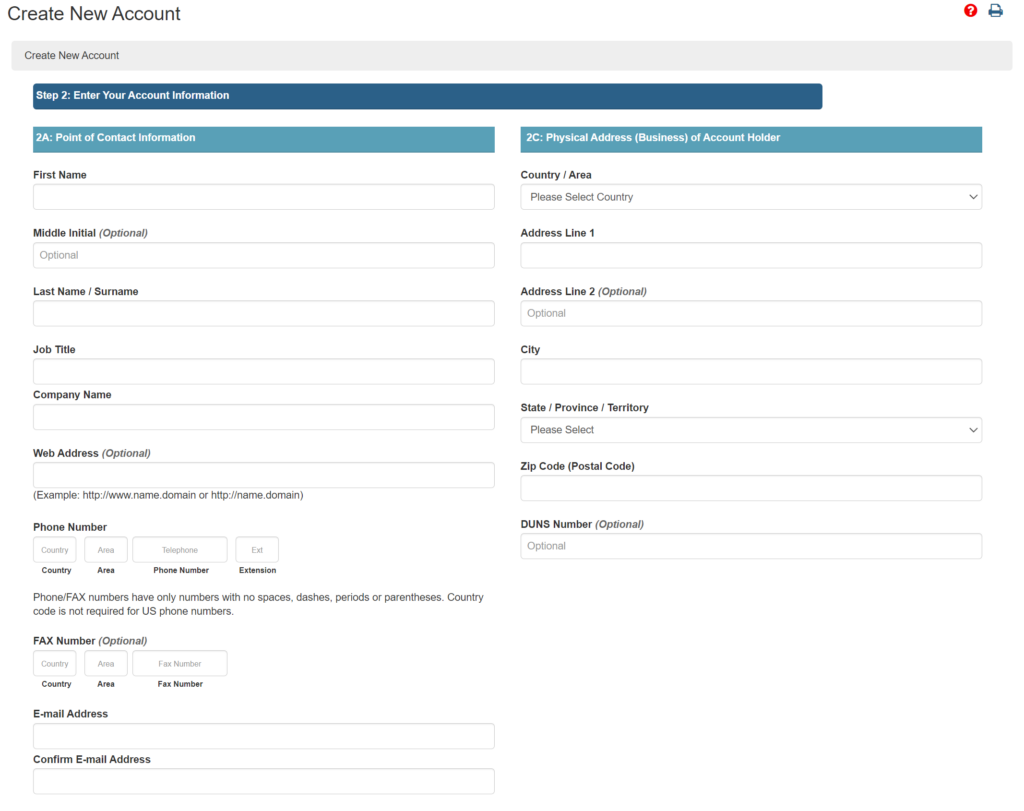
The US Agent is not required to be a legal entity, but you will need to enter a “Company Name.” There is no place to enter an EIN, and DUNS number is optional. Here’s a screen capture of the account creation form below.
You should also consider adding your agent to your Approved Supplier List (i.e., LST-003). If you do not already have a procedure for Supplier Quality Management (i.e., SYS-011), Medical Device Academy has a procedure available for purchase that includes a template for review and approval of new suppliers (i.e., FRM-005) and a template for an Approved Supplier List (i.e., LST-003). The FDA US agent doesn’t need a quality system, but they should be able to demonstrate competency in US FDA device regulations with their resume and/or training records. Specifically, competency should include 21 CFR 820, 803, 806, 830, and 807. In the future, your US agent must also be competent in ISO 13485:2016. FDA inspectors are expected to request evidence of an agreement between your company and the US agent. The inspector will also review your records for qualification, approval, and ongoing evaluation of the US agent as a supplier during FDA inspections. Ideally, your agent has been directly involved in previous FDA inspections, and they can prepare you by conducting a mock-FDA inspection.
What does the FDA do to qualify US agents?
The FDA does very little to qualify a US agent. The only thing the FDA “does” is to send an automated email to the FDA US agent when you submit your initial establishment registration or renew your FDA registration. The email subject line is “ACTION REQUIRED: U.S. Agent Assignment Notification.” The email is sent from “reglist@cdrh.fda.gov.” Your agent must ensure their email client has identified this email as a “safe sender” to prevent the email from ending up in a spam folder. For medical devices, there is no requirement for the US agent to submit any other proof to the FDA.
What is an “Action Required” email?
Below is an example of the “Action Required” email that the FDA sends to FDA US agents immediately after your registration and listing is completed by a foreign firm.

Your FDA US agent will receive an automated email from the FDA seconds after you complete your registration for an initial FDA establishment registration or the renewal of your FDA establishment registration. The agent then has ten (10) days to log in to their FURLS account and confirm that they are willing and able to serve as your company’s US agent. The email notifying your US agent includes the following language:
“If you are the U.S. Agent for this establishment, select “Yes”, and click “Submit”. If you are not the U.S. Agent for this establishment, select “No”, and click “Submit”. You must confirm you are the U.S. Agent within 10 business days. If you do not confirm that you are the U.S. Agent within 10 days, the system will automatically cancel your Receipt Code and remove the U.S. Agent information associated with the foreign establishment.”
Suppose the agent does not confirm their role within ten business days. In that case, the FDA will automatically email your company that the agent did not confirm their role. If you select a more reliable US agent, you must resubmit the request for the same person or a new person.
If you have additional questions or need a US agent, please contact Medical Device Academy.
FDA US Agent – What do they do? Read More »