Wolfgang Huber presented a free Webinar on how to create a traceability matrix for 510k software documentation on Wednesday, September 13th, 2017.
Providing a traceability matrix is one of the documentation requirements of the FDA for a 510k submission of medical devices containing software, including software as a medical device (SaMD). Most companies use a spreadsheet to do this, but you deal with so many items that a traceability table would be huge. For the purpose of this webinar and our 510k workshop in Amsterdam, we can use a spreadsheet. However, when you have 100+ items in each category of your matrix, the matrix becomes quite unusable. Wolfgang will demonstrate how the same information you traditionally placed in a spreadsheet can be imported into a database for greater utility.
When is the Traceability Matrix Webinar by Wolfgang Huber?
This traceability matrix webinar was presented by Wolfgang Huber on Wednesday, September 13, 2017. It’s free to download as long as you ask Wolfgang a question. As new questions are submitted I will create blogs to answer your questions and add the questions to our 510k FAQs page. In order to register for the traceability matrix webinar please fill in and submit the form above. You will receive an email confirmation including instructions on how to view and participate in the webinar via Zoom. You will also receive a link to download a recording of the webinar and the native slide deck after the live webinar. Additional questions can be asked at any time by sending me an email or scheduling a call on my contact us page.
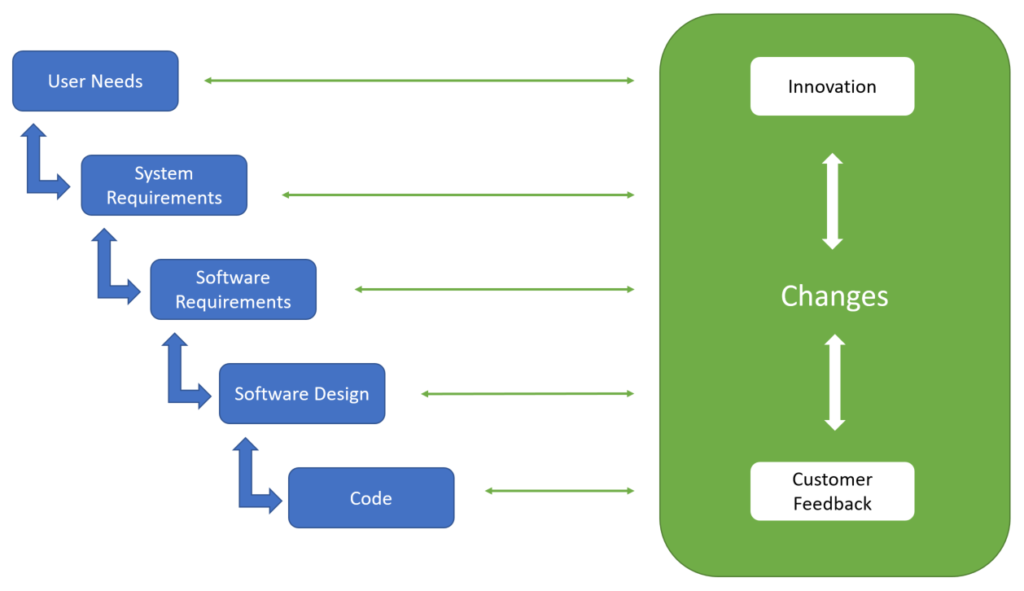
Contents of Traceability Matrix Webinar
In this webinar, Wolfgang will explain the traceability requirements for software, and how the requirements can be efficiently applied in agile software development projects. The main challenges seen today are:
-
The waterfall approach to device design documentation is not ideally suited to the agile product development model. Longer, predefined development phases in the waterfall approach (e.g., planning, design, development, verification, and validation) are replaced by shorter, iterative development phases called sprints. Maintaining traceability in design documents becomes a major burden because system and software requirements evolve in parallel with design and code.
-
Traceability from architecture, to design, to code and interfaces is hard to establish and less useful with state-of-the-art software development tools and best practices.
-
Software changes, specifically for newer, software-centric devices, can be done faster and more efficiently today if the management of design change control is optimized to limit the requirements for re-verification and re-validation.
About Your Instructor Wolfgang Huber
Wolfgang Huber is the Co-Founder of Matrix Requirement Medical. He is a software engineer that will be speaking on IEC 62304 and the requirements for software documentation in a 510k submission. His company developed a web-based application to help medical devices organize documentation (requirements, specifications, use cases, tests, risk analysis) for their Design History File (DHF). The software eliminates the need to exchange word documents, maintains version control, and automatically creates a traceability matrix. The company website includes testimonials for customers that use the software for creating their 510k submission documents.


