Stay compliant with ISO 13485:2016 and 21 CFR 820.50 with our updated purchasing procedure. Download now and simplify your process.
Purchasing Procedure Webinar
This purchasing procedure is written to ensure that goods and services purchased by your company meet customer and regulatory requirements and that purchasing is controlled appropriately for the risk of your products. The 5-page procedure complies with for ISO 13485:2016, Clause 7.4.1 and 21 CFR 820.50. The procedure does not include the supplier quality management requirements.
When is the live webinar scheduled for the Purchasing Procedure?
The live webinar is scheduled for Monday, November 11, 2024 @ 10:30 a.m. ET. If you purchased the procedure before November 11, you will receive login information to participate in the live webinar. The webinar will be hosted on Streamyard.com. If you are unable to participate in the live webinar, please send us your questions in advance so that we can be sure to address your questions in the live webinar. You can download the recording from the Dropbox folder after the live webinar and watch it as many times as needed.
Contents Sold with the Purchasing Procedure
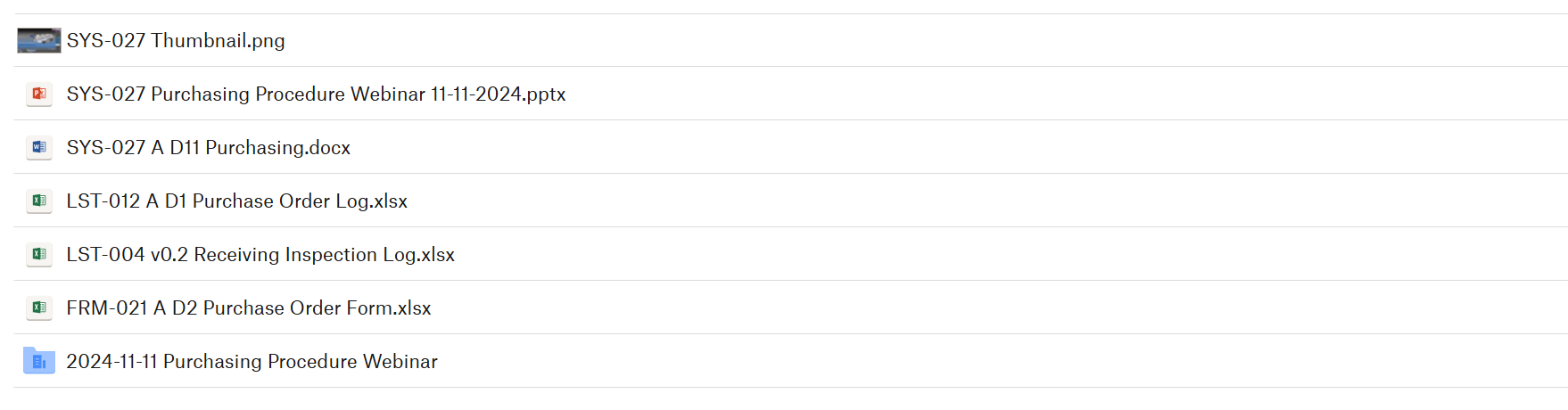
Purchasing Product Webinar Bundle available for $299.00:
Please note: This product will be delivered to the email address provided in the shopping cart transaction. After the transaction is verified, please check your email for the download. To view all available procedures click here.
VIEW OUR PROCEDURES – CLICK HERE OR IMAGE BELOW:
About Your Instructor

Rob Packard is a regulatory consultant with ~25 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Rob was a senior manager at several medical device companies—including the President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009 to 2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Rob’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510k submissions. The most favorite part of his job is training others. He can be reached via phone at +1.802.258.1881 or by email. You can also follow him on YouTube, LinkedIn, or Instagram.


