Participant Screening & Data Collection Forms webinar ($79)
In this human factors training webinar, you will use this form when your conducting either summative or a formative usability study. Matthew Walker will be the instructor for this webinar.
Please note: These products will be delivered to the email address provided in the shopping cart transaction. After the transaction is verified, please check your email for the download.
When is the Participant Screening & Data Collection Forms webinar?
This webinar is a 25-minute recording you can purchase on-demand and watch the training as often as you wish. If you need help preparing a usability engineering file (UEF) for your device, we can help you on an hourly basis. Please contact Lindsey Walker for a quote.
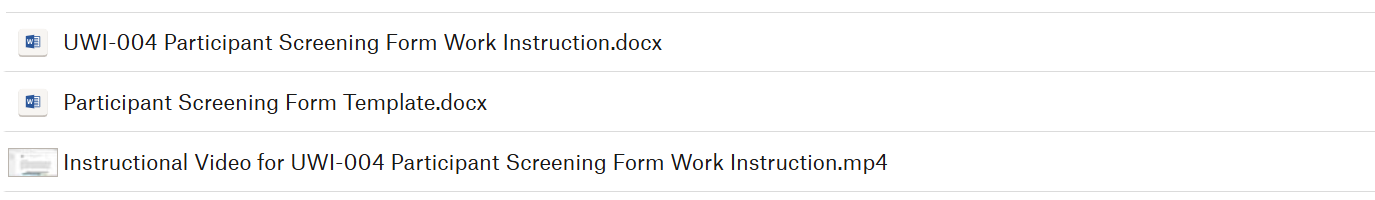
What you will receive in the Participant Screening & Data Collection Forms webinar:
Other Usability Engineering / Human Factors Training
- Human Factors Training Series – $950
- Formative Usability Testing Webinar & Template Bundle – $79
- Use Error and Abnormal Use Decision Tree Training – $79
- Use Specification Template & Webinar Bundle – $79
- Task Analysis Template & Webinar Bundle – $129
- Use-Related Risk Analysis (URRA) Template & Webinar Bundle – $299
- Summative Usability Testing Protocol & Webinar Bundle – $199
- Summative Usability Testing Report – Live-streaming Free Webinar
About the Author
 Matthew Walker – QMS, Risk Management, Usability | Human Factors Engineering, Cybersecurity & DFIR
Matthew Walker – QMS, Risk Management, Usability | Human Factors Engineering, Cybersecurity & DFIR
Matthew brings a unique background as a former Firefighter/EMT and Rope Rescue Tech with experience in OSHA and NFPA regulations. For the better part of a decade, he has worked as a Technical/Medical Writer and Lead Auditor. He holds degrees in Fire Science and Computer Forensics and Digital Investigations, graduating Summa Cum Laude from Champlain College. Matthew is also an active member of several academic honor societies including Omicron Sigma Sigma’s Order of the Sword and Shield. His professional focus includes Human Factors Engineering, Risk Management, and Cybersecurity with a special interest in applying Digital Forensics and Incident Response (DFIR) practices to medical technology. He combines regulatory expertise with technical insige to strengthen both product safety and oranizational resiliance. He can be reached by email. You can also follow him on LinkedIn or YouTube.
