In this hazard identification webinar you will learn multiple methods of identifying hazards, including use-related and software hazards.
Hazard Identification Webinar ($79)
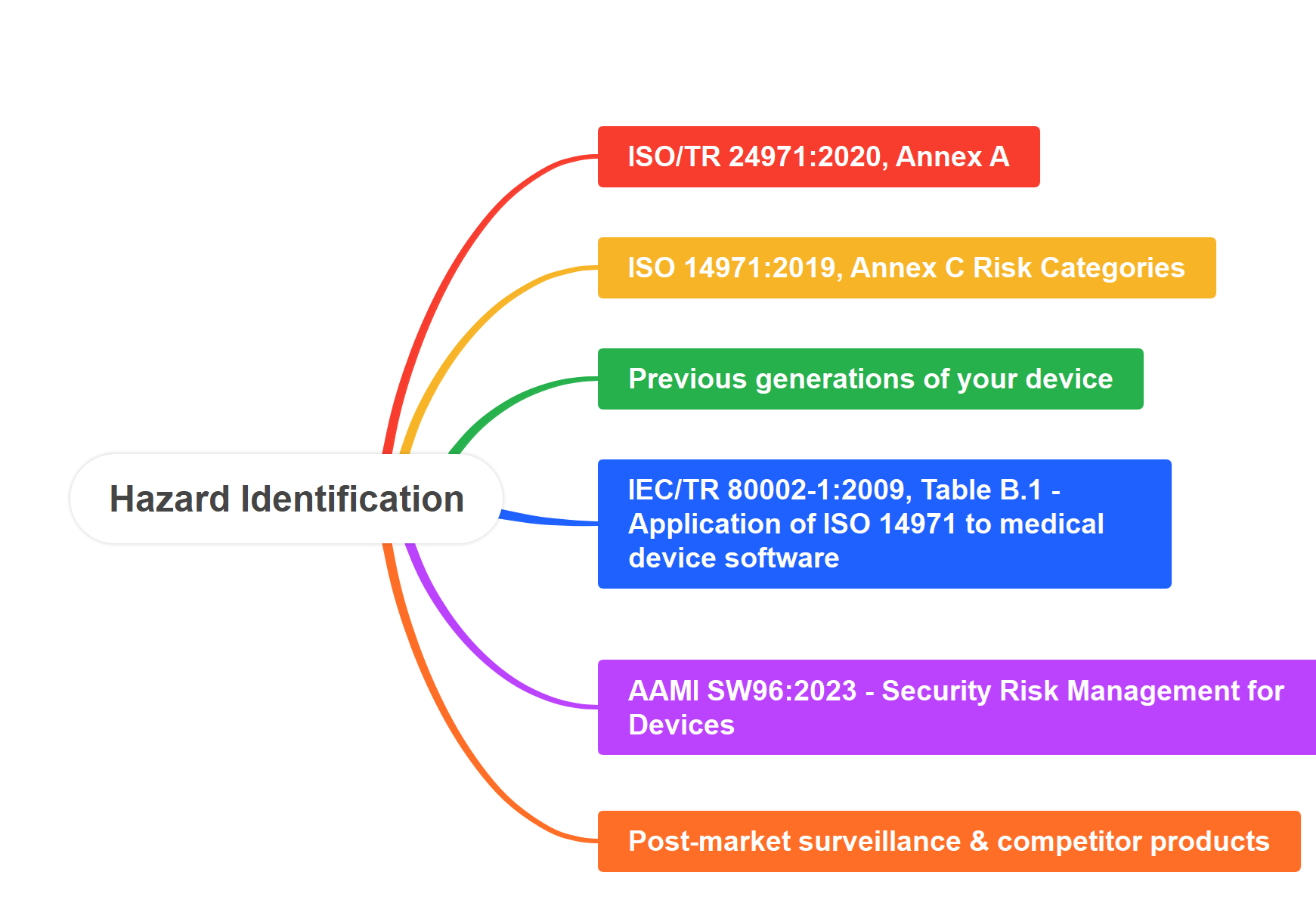
There are multiple methods for hazard identification, but most companies stop after using one method. In this webinar you will learn multiple methods for identifying hazards. The webinar training includes demonstrating hazard identification for multiple device examples. You will also learn how to identify use-related risks and software risks. Webinar attendees will receive a copy of our hazard identification template as well.
Please note: These products will be delivered to the email address provided in the shopping cart transaction. After the transaction is verified, please check your email for the download.
When is the Hazard Identification Webinar?
This webinar is scheduled for August 29, 2024 @ 10:30 a.m. ET. You can purchase it on-demand and watch the training as often as you wish. If you need help preparing your risk management file or facilitating hazard identification for your device, we can help you on an hourly basis. Please contact Lindsey Walker for a quote.
What you will receive in the Hazard Identification Webinar:
- Hazard Identification Template
- Login information to live webinar (if purchased on or before August 29, 2024)
- Recording of live webinar
- Native PowerPoint Slide Deck
About Your Instructor
Rob Packard is a regulatory consultant with ~25 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Rob was a senior manager at several medical device companies—including the President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009 to 2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Rob’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510k submissions. The most favorite part of his job is training others. He can be reached via phone at +1.802.281.4381 or by email. You can also follow him on YouTube, LinkedIn, or Twitter.