Do you need help completing your initial FDA registration and listing for a medical device? Watch our video to learn how.
The two most common situations for when a company needs to register its establishment with the FDA are 1) when the company is a contract manufacturer and producing a finished device for the first time, and 2) when the company is a specifications developer that recently received a 510k and is about to begin distribution of the newly cleared product. If your company is a specification developer, and you have not yet submitted your first 510k, then you must complete your Medical Device User Fee Cover Sheet first. If you have already received 510k clearance, or your device is exempt from 510k clearance, this article and the associated video will help you complete your FDA registration and listing.
Small Business Status does not apply to FDA registration
Most first-time 510k submissions are from small companies. If your company has gross receipts of less than $100 million, you should apply for status as a small business by completing FDA Form 3602 (for US-based companies) or FDA Form 3602A (for foreign companies)–along with your company’s tax return for the previous year. You should apply every year on August 1st. The qualification process takes 60 days, and you never know when you might need to submit a 510k for a device modification. Qualifying for small business status saves substantially on FDA submission fees. The FDA’s review and decision regarding your application for small business status require 60 days, and the status expires each year on September 30th. If you want additional information about small business qualifications, we created a webpage dedicated to this topic.
Medical Device User Fee Amendment (MDUFA)
A few weeks before you submit your first 510k to the FDA, it is recommended that you create a new account for the user fee website and make your Device Facility User Fee (DFUF) payment. This is the website you must access to pay the 510k submission fee. If you are taking advantage of small business status, you will need the Small Business Decision Number you received in the FDA decision letter in response to FDA Form 3602A. Small and large businesses should follow the directions in the guidance document to set up a new MDUFA account.
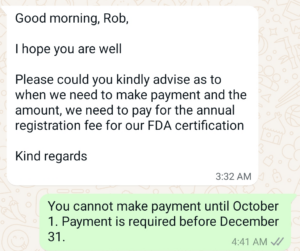
Once the user fee account has been created, you need to complete a 510k user fee cover sheet. The screen capture posted below was a Whatsapp communication with one of Medical Device Academy’s clients. The FDA User Fee is paid directly by the company to the FDA. You have three payment options: 1) ACH transfer (from US Banks only), 2) Credit Card (your credit card may have a limit below the amount of the FDA User Fee), and 3) wire transfer. The FDA offers multiple ways to pay the user fee for submission or FDA registration. The client below was specifically asking about annual FDA registration.
 After you submit your 510k and receive your 510k clearance letter, you may begin marketing and distributing a product. Once a company starts distributing a new product, the company has 30 days to register the facility and list each device with the FDA. Before registering with the FDA, you must also make a second DFUF payment for the establishment registration fee of $11,423 for FY 2026 (a 23.1% increase from FY 2025). There is no discount for small business status when paying the FDA registration fee, and the fee is not prorated. Discounts are only available for submission fees (e.g., the 510k user fee). The annual FDA registration fee must be paid for each facility registered between October 1 and December 31. Your registration will become inactive if renewal fees are not paid on time. If your device requires 510k clearance, you will not be able to complete the listing process until you obtain clearance. If the device is your first product, you do not need to register until you receive the 510k clearance.
After you submit your 510k and receive your 510k clearance letter, you may begin marketing and distributing a product. Once a company starts distributing a new product, the company has 30 days to register the facility and list each device with the FDA. Before registering with the FDA, you must also make a second DFUF payment for the establishment registration fee of $11,423 for FY 2026 (a 23.1% increase from FY 2025). There is no discount for small business status when paying the FDA registration fee, and the fee is not prorated. Discounts are only available for submission fees (e.g., the 510k user fee). The annual FDA registration fee must be paid for each facility registered between October 1 and December 31. Your registration will become inactive if renewal fees are not paid on time. If your device requires 510k clearance, you will not be able to complete the listing process until you obtain clearance. If the device is your first product, you do not need to register until you receive the 510k clearance.

FDA Registration and Listings System (FURLS) Database
The FURLS database is a separate database where companies register facilities and list devices with the FDA. The FURLS account ID and password used for FDA registration of your facility is separate from the user name and password for the user fee website used for the DFUF payment. After you pay the annual registration user fee, you will receive the following information via email: Payment Identification Number (PIN) and Payment Confirmation Number (PCN). You will need this information to complete the registration in the FURLS database.
To create a new FURLS account, you access the following website. This new account should only be created if your company does not already have one, and the person creating the account should be a trusted manager with the authority to designate sub-accounts when needed. Creating a new account will result in issuing an account ID, and you must select a password during the process. You need this information for logging into the system in the future. The FDA also has an account management page if you already have an FDA registration and listing account and need help managing it or making changes.
FDA Registration and Listing – Additional Resources
The FDA created a webpage explaining medical device FDA registration and listing, but the following page is the place I recommend that most companies begin reading. If you want Medical Device Academy to help you with FDA registration, we provide assistance with registration and listing as a consulting service (i.e., $800 for initial registration of your first product, and $600 for annual renewal of registration). Registration and listing is also included with our US Agent services. You can purchase these services by using our calendly link for registration and listing assistance.
If you want additional training on registering and listing your facility with the FDA, please visit the updated CDRH Learn webpage: (Click on “Start Here/The Basics”). The FDA offers a “post-test” and certificate for anyone completing the post-test. I recommend completing this training before setting up a new account and anyone responsible for updating the FDA registration and listing information.
