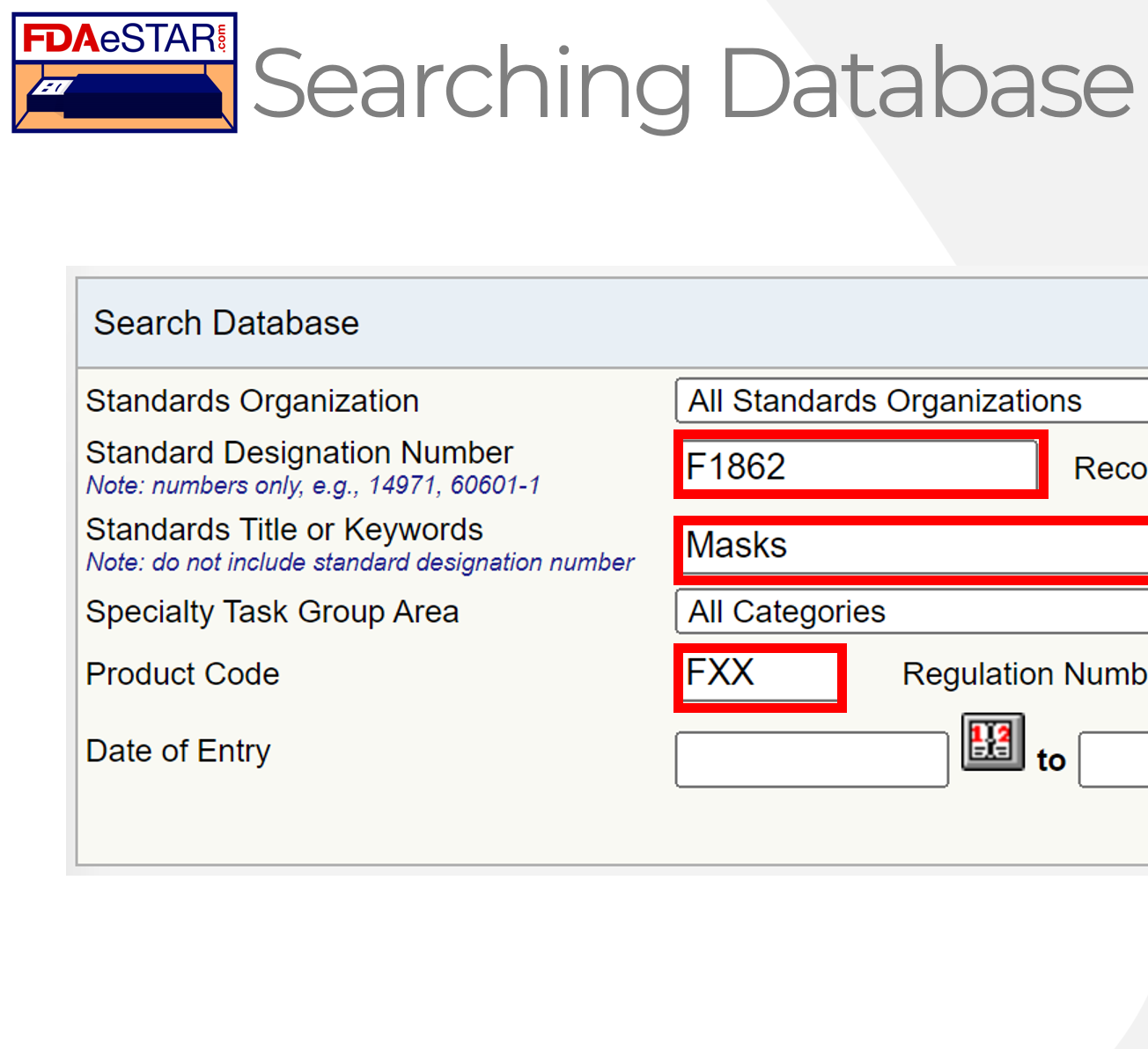
This webinar explains how to use the FDA Consensus Standards Database to determine which standards apply to your device.
Consensus Standards Webinar ($79)
This webinar is included in our 510k Course series. You will be notified of the live webinar by email if you have already purchased the course. If you purchase the 510k Course series later, you can request a credit for your prior purchase.
Please note: This product will be delivered to the email address provided in the shopping cart transaction. After verifying the transaction, please check your email for the download (including your spam folder).
When is the Consensus Standards Webinar?
The live webinar is scheduled for March 14, 2024 @ 10:30 am ET. If you purchase the webinar before the live presentation, you will receive a link to participate in the live webinar via Zoom.
What you will receive
- Access to download the recording of the webinar from our Dropbox folder
- Native slide deck for this webinar
There will be 25-35 slides during the presentation. The presentation will be 45-50 minutes in duration. All deliveries of content will be sent via AWeber emails to confirmed subscribers.
Why you should register for the Consensus Standards Webinar?
This webinar explains how to use the FDA Consensus Standards Database to determine which standards apply to your device. The webinar also explains the difference between declaring conformity and the general use of a standard. We explain ASCA certification in the video and teach you how to look up standards in the 510k process.
About Your Instructor
Rob Packard is a regulatory consultant with ~25 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Rob was a senior manager at several medical device companies—including the President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009 to 2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Rob’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510(k) submissions. The most favorite part of his job is training others. He can be reached via phone at 802.281.4381 or by email. You can also follow him on YouTube, LinkedIn, or Twitter.